Download PDF
Can a novel technology reveal retinal damage earlier than currently available imaging modalities? Researchers have yet to prove that it can, but they are pursuing leads that fluorescence lifetime imaging ophthalmoscopy (FLIO) may be useful for detection of hydroxychloroquine (HCQ; Plaquenil) toxicity in a retina that appears otherwise healthy.1
“FLIO is a novel technology that is not yet widely known, but it has already revealed disease-related patterns for age-related macular degeneration and other retinal conditions,” said Lydia Sauer, MD, at the John A. Moran Eye Center in Salt Lake City, Utah. “We believe that it has great potential to enhance the diagnosis of retinal diseases by detecting metabolic changes in the retina, before overt damage occurs.” Other imaging modalities, including optical coherence tomography (OCT), describe changes only after retinal damage is manifest.
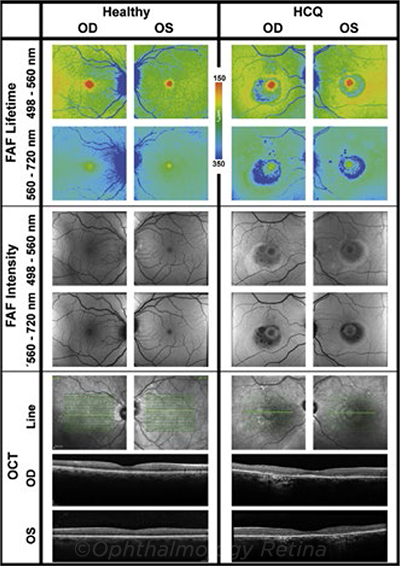 |
COMPARISON. Fundus autofluorescence lifetime and intensity images, as well as OCT imaging, from a healthy subject and a patient with severe HCQ toxicity.
|
What FLIO may tell us. This noninvasive imaging technology measures the span of time that naturally occurring retinal fluorophores glow, following excitation with a laser pulse. This is known as the autofluorescence lifetime.
Dr. Sauer and her colleagues harnessed FLIO to measure retinal toxicity from HCQ, which is well-known for its ability to cause retinal damage.
Study specifics. The researchers used a modified Spectralis OCT (Heidelberg Engineering) to measure fluorescence lifetimes in 58 patients. Of these, 12 had definite HCQ toxicity; eight were clinically normal and considered at low risk, as they had been on HCQ for less than five years; 16 were clinically normal but considered high risk, as they had been on the drug for more than five years; and 22 were age-matched healthy controls.
All of the patients with definite HCQ toxicity showed significantly prolonged FLIO lifetimes in regions of damage. Of the clinically normal patients, nine of the 16 in the high-risk HCQ group (56%) and two of the eight in the low-risk group (25%) showed prolonged autofluorescence lifetimes in a pattern suspicious for HCQ toxicity. No such patterns were observed in the healthy controls.
Next steps. Dr. Sauer stressed that researchers are unsure about the metabolic and structural origins of the altered FLIO lifetimes. Thus, they plan to monitor their cohort of “suspicious” patients to determine whether FLIO findings suggestive of HCQ toxicity evolve to overt damage visible with established imaging modalities, she said. “This would prove our hypothesis and may allow us to change individual treatments based on FLIO findings to preserve eyesight.”
—Miriam Karmel
___________________________
1 Sauer L et al. Ophthalmol Retina. Published online May 2, 2019.
___________________________
Relevant financial disclosures—Dr. Sauer: Novartis: C; Tesseract: C. Heidelberg Engineering provided nonfinancial support.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chen National Institute on Aging: S; Quark Pharmaceuticals: C.
Dr. Drack Chakraborty Foundation: S; Canadian FFB: S; NIH: S; ProQr; S; Retrophin: S; Spark: S; Vision for Tomorrow: S.
Dr. Lass Alcon: S; Eversight Ohio: C; Transcend Medical: S.
Dr. Reiss Aerie: L; Alcon: C,L,S; Allergan: C,S; Bausch Medical: L; Glaukos: L; Haag-Streit: L; Santen/InnFocus: C,S.
Dr. Sauer Novartis: C; Tesseract: C.
Dr. Utz Retrophin: S; Springer Publishing: P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review