Download PDF
Fancine Filbert* was at her wits’ end. The 60-year-old attorney was fed up with prism glasses that didn’t correct her double vision. For 3 years she had seen various providers for intermittent binocular diplopia that was getting more bothersome. The story was always the same: After carefully explaining her predicament, she’d undergo a battery of tests, only to emerge with a prescription for new prism glasses. Although these prescriptions seemed to correct her double vision in the clinic, her diplopia always returned a short time later.
We Get a Look
Frustrated, Ms. Filbert described nearly constant diplopia with 2 distinct images that were diagonal to each other in all gaze directions. She said that if she closed either eye, her diplopia completely disappeared. Her medical history was notable only for well-controlled hypertension. She had mild myopia in both eyes (–1.50 sphere) and no other past ocular history.
On exam, best-corrected visual acuity measured 20/20, color vision was normal, and confrontation visual fields were full in each eye. Amsler grid testing revealed a central area of metamorphopsia in the left eye. Her pupils were isocoric in all lighting conditions and responded briskly to light. There was no afferent pupillary defect. Ocular motility was full in both eyes, and there was no nystagmus. At the slit lamp, we noted mild nuclear sclerotic cataracts in both eyes. Cranial nerves III through XII were intact. There was no baseline ptosis, fatigable ptosis, or orbicularis oculi weakness.
Alignment testing with alternate-cover and single Maddox rod techniques revealed a comitant 5–prism diopter (PD) exotropia and a 2-PD right hypertropia. Double Maddox rod testing showed no torsion in either eye.
Her prior diagnostic workup included magnetic resonance imaging of the brain and orbits, thyroid function studies, and acetylcholine receptor antibody testing for myasthenia gravis, which were all normal. Her most recent pair of ineffective prism glasses had 1-PD base-down and 2-PD base-in in the right eye and 1-PD base-up and 2-PD base-in in the left eye.
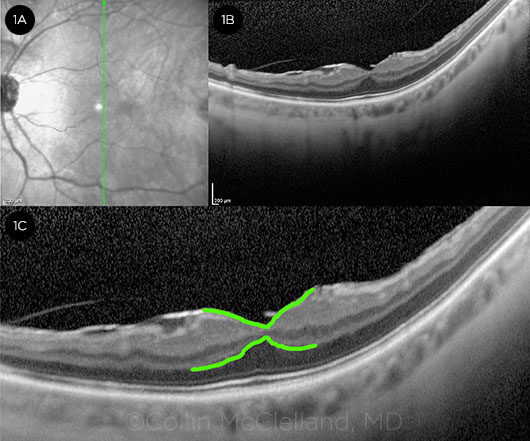
Funduscopic exam was normal in the right eye and showed a faint epiretinal membrane (ERM) in the left eye’s central macula. Spectral-domain macular optical coherence tomography (OCT) of the left eye revealed an ERM distorting the foveal pit architecture and mild vitreomacular traction (Fig. 1).
 |
|
IMAGING. (1A) Infrared image of the patient’s left eye: The vertical green arrow indicates the cross-section corresponding to the OCT images shown. (1B) Macular OCT demonstrates mild vitreomacular traction and ERM with distortion of the normal foveal contour. (1C) High-magnification OCT image with the inner retina and outer plexiform–outer nuclear layer junction traced in bright green to highlight the tractional forces exerted on the fovea.
|
Making the Diagnosis
We suspected that Ms. Filbert’s diplopia was related to her subtle ERM. A few features of her presentation were clues to the diagnosis.
First, the patient described unilateral metamorphopsia on Amsler grid testing of the left eye that was consistent with her ERM. Also, without prisms she was able to achieve binocular fusion of a single 20/70-size white optotype when the exam room lights were extinguished, yet her diplopia recurred within seconds when the lights were turned back on.
These findings, along with her prism-refractory binocular oblique diplopia, are diagnostic of dragged-fovea diplopia syndrome (DFDS).
Discussion
In 1980 Burgess et al. first described DFDS, also known as foveal displacement syndrome, in a cohort of patients with binocular diplopia related to subretinal neovascular membranes.1 The syndrome occurs when underlying macular pathology such as a choroidal neovascular membrane (CNVM), ERM, or tumor causes 1 or both foveae to become mechanically displaced (“dragged”) relative to each other. Diplopia occurs when the magnitude of displacement is such that the foveae no longer occupy corresponding retinal areas. These patients experience central binocular diplopia due to competition between central (foveal) and peripheral retinal fusion. Since corresponding binocular central and peripheral retinal points cannot be stimulated simultaneously due to the displaced fovea, the stronger peripheral fusion drive wins out, resulting in persistent central binocular diplopia.
The majority of patients with DFDS present with complaints of vertical or oblique binocular diplopia, often accompanied by unilateral metamorphopsia. Apart from a history of known macular pathology, clues to DFDS include a history of prior ocular surgery, including cataract extraction, prior retinal laser treatment, or vitrectomy. Most patients with DFDS exhibit a small-angle, relatively comitant vertical strabismus. In a series of 83 patients with DFDS, De Pool et al. found the average vertical misalignment to be 2.7 PD, and many patients also had small comitant eso- or exodeviations.2 Patients with DFDS have difficulty with prism adaptation. Despite the initial disappearance of diplopia for some patients with prism trials in the office, most patients experience a slippage of central fusion and recurrence of diplopia within seconds to minutes as peripheral fusion mechanisms overcome central fusion.2
DFDS theoretically can be caused by any macular pathology that mechanically displaces the fovea, although ERM is the most common cause.2 A history of prior ocular surgery or trauma should cue the clinician to look carefully for an ERM.
Pinning It Down
Nearly all patients with DFDS will exhibit visible macular pathology on exam. As seen in our case, OCT of the macula can be helpful in augmenting the funduscopic exam, since structural causes of DFDS can be subtle.
Amsler grid testing is a useful screening tool for patients presenting with complaints of binocular diplopia. While the majority of patients with DFDS describe unilateral metamorphopsia on Amsler grid testing, some patients will not volunteer metamorphopsia when providing their history. Abnormal Amsler grid testing in a patient with binocular diplopia should raise the suspicion for DFDS and prompt careful investigation for underlying macular pathology.
Finally, the lights on–lights off test can be used to confirm a diagnosis of DFDS in patients with suggestive clinical features. In this test, the patient is asked to fixate on a single white 20/70-20/100 letter on a black background. A patient with DFDS sees the single white letter as 2 distinct images centrally (often vertically or obliquely oriented to each other) with the room lights on. When the room lights are turned off, however, the patient reports fusion of the doubled letter into a single letter (Fig. 2). This test works on the principle that with room lights on, binocular vision is driven by stronger peripheral fusion mechanisms that dominate over central fusion. Since DFDS is caused by rivalry between the competing central and peripheral fusion, removing peripheral retinal stimuli (by shutting off all room lights) will resolve diplopia.
 |
|
THE LIGHTS ON– LIGHTS OFF TEST. (2A) The setup for the test: A single white optotype (20/70-20/100) is shown against a black background. (2B) A simulation of what the patient with DFDS might describe with room lights on: central oblique diplopia with some distortion of the image arising from the abnormal fovea. Note that fusion has occurred in the periphery, but central fusion cannot be achieved. (2C) A simulation of what a patient with DFDS would describe when the room lights are turned off: rapid central fusion of the diplopic image due to the absence of peripheral retinal stimuli in the dark environment.
|
Treatment
Although vitrectomy surgery with ERM peeling seems a plausible treatment for ERM-related DFDS, the majority of patients who undergo ERM peeling after the diagnosis of DFDS experience little or no improvement in diplopia.2 Furthermore, ERM peeling may unmask or lead to the development of DFDS in a subset of patients because of an improvement in visual acuity following surgery, effectively unblurring the diplopic image. A discussion of possible postoperative DFDS and difficult-to-treat diplopia should be a part of the preoperative risk discussion in all patients considering ERM-peeling procedures.
The relatively small-angle comitant strabismus that classically accompanies DFDS is notoriously refractory to prism correction, although a minority of patients will respond favorably. While prism trials in the clinic may initially relieve diplopia, the effect is typically short-lived, and central diplopia returns within seconds to minutes. For this reason, providers prescribing ground-in prism to DFDS patients should have them try the prism prescription for several minutes in clinic or consider an initial temporary Fresnel prism trial.
Monovision glasses can be helpful in treating DFDS patients. Although central diplopia may not disappear completely, some patients find it easier to ignore with one eye blurred.
Monocular occlusion is also an option for patients and can be accomplished with a variety of materials ranging from Scotch tape to Bangerter foils to occlusive contact lenses.3
Conclusion
Clinical features suggestive of DFDS include vertical or oblique binocular diplopia with a corresponding small-angle vertical strabismus, unilateral or bilateral metamorphopsia, a history of multiple unsuccessful attempts at prism correction, and a history of ERM or CNVM. In DFDS suspects, a careful fundus examination with Amsler grid testing or macular OCT may be helpful in identifying causative macular pathology. A positive lights on–lights off test in the office confirms the diagnosis and may help avoid future unsuccessful ground-in prism prescriptions.
___________________________
* Patient name is fictitious.
___________________________
1 Burgess D et al. Arch Ophthalmol. 1980;98(2):311-317.
2 De Pool ME et al. Ophthalmology. 2005;112(8):1455-1462.
3 Silverberg M. Arch Ophthalmol. 1999;117(7):900-903.
___________________________
Dr. Chaon is a second-year resident and Dr. McClelland is an assistant professor of neuroophthalmology; both are with the Department of Ophthalmology and Visual Neurosciences at the University of Minnesota. The authors would like to acknowledge Erik van Kuijk, MD, PhD, for his assistance with this case. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.