By Richmond Woodward, BA, Abdallah Mahrous, MD, Mrinali P. Gupta, MD, and Sarah H. Van Tassel, MD
Edited By: Steven J. Gedde, MD
Download PDF
Tatiana Ivanov,* a 79-year-old Albanian woman now living in New York City, grimaced and held her hand over her right eye. She reported that the vision in that eye had become blurry 4 days earlier. Two days after the onset of blurred vision, she began to experience severe pulsating eye pain and worsening vision. Now, she said that she could see only “fog” with her right eye.
Ms. Ivanov described several episodes of self-limited painless vision loss in the right eye that had occurred over the past year. Each of these lasted a few days before her vision returned to normal. Apart from these episodes, she had enjoyed excellent vision in her right eye since undergoing cataract surgery 5 years ago.
Emergency Department Findings
Ms. Ivanov was first seen in the ED, where her visual acuity was recorded as light perception in the right eye and 20/25 in the left eye. Intraocular pressure (IOP) was 70 mm Hg in the right eye and 12 mm Hg in the left eye. Slit-lamp examination showed profound microcystic edema of the right cornea, limiting visualization of intraocular structures. B-scan ultrasonography revealed vitreous hemorrhage.
Intravenous acetazolamide and serial IOP-lowering drops were administered, and her IOP improved to 26 mm Hg prior to discharge from the ED.
We Get a Look
The following day, Ms. Ivanov presented at our office feeling much better; her eye pain had resolved, and IOP was 12 mm Hg in the right eye. Additional history revealed that she had systemic hypertension, dyslipidemia treated with statins, and exfoliation syndrome (XFS).
Gonioscopy showed an open angle in the right eye with hyphema inferiorly. We saw no neovascularization of the angle. The anterior chamber was filled with 4+ cells; although both pigmented and nonpigmented types were present, the pigmented cells were more numerous. A well-centered single-piece posterior chamber intraocular lens (IOL) was noted.
Differential Diagnosis
The profusion of pigmented cells in the anterior chamber coupled with the vitreous hemorrhage suggested that intraocular bleeding was the most likely cause of the marked IOP elevation. Our differential diagnosis included hemolytic glaucoma and ghost cell glaucoma, both of which can develop following vitreous hemorrhage.
In hemolytic glaucoma, hemoglobin-laden macrophages produce obstruction of the trabecular meshwork, and red cells predominate in the anterior chamber. In ghost cell glaucoma, degenerated red blood cells that have lost their intracellular hemoglobin cause the outflow obstruction, and small, khaki-colored “ghost cells” are noted in the anterior chamber. Vitreous hemorrhage is usually present for 1 to 3 months before red blood cells degenerate into ghost cells. Given the sudden onset of Ms. Ivanov’s blurry vision 4 days prior to presentation and the predominance of pigmented cells in the anterior chamber, hemolytic glaucoma was the most likely explanation for the elevated IOP.
However, the etiology of the vitreous hemorrhage remained unclear. Although Ms. Ivanov had a history of hypertension, there were no signs of hypertensive retinopathy in the fellow eye. Our working diagnoses were acute hemorrhagic posterior vitreous detachment or retinal vein occlusion, and consultation with a retina specialist was arranged. In the meantime, IOP-lowering therapy was continued.
Next Steps
Retina consult. Ms. Ivanov saw the retina specialist 8 days after presentation. The vision in her right eye had improved to 20/50, and the IOP was 12 mm Hg. The anterior chamber was still filled with pigmented cells, and the vitreous hemorrhage was clearing. Ms. Ivanov’s daughter, who accompanied her to the appointment, recalled that her mother’s blood pressure had been very high in the weeks before the severe eye pain started. However, Ms. Ivanov’s retinal examination and fluorescein angiography showed no evidence of retinal vascular occlusion to implicate hypertension as the etiology of the bleeding. The hyphema was followed to resolution, and the vision in the right eye improved to 20/25.
Optic nerve head exam. Once the vitreous hemorrhage had cleared, we could examine the optic nerve head. In the right eye, we found a vertical cup-to-disc ratio of 0.6 with thinning of the inferior neuroretinal rim; in the left eye, we observed a cup-to-disc ratio of 0.3. Optical coherence tomography (OCT) demonstrated intact and symmetric retinal nerve fiber layer thickness and inferior ganglion cell loss in the right eye. Automated perimetry was performed reliably in both eyes. Several superonasal pixels were present in the right eye, raising concern for an early nasal step. The left eye testing was full.
New Findings
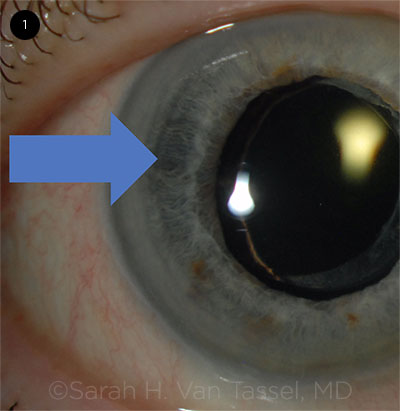
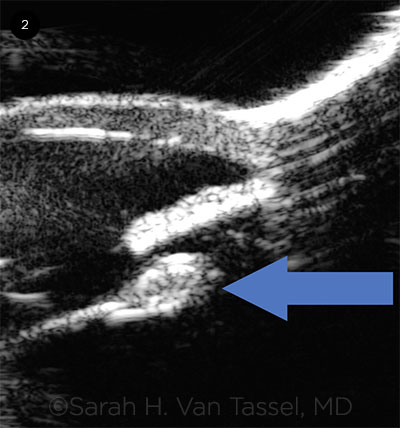
About 1 month after the initial presentation, Ms. Ivanov returned for follow-up, reporting another episode of vision loss—which had since resolved—in the previous week. Slit-lamp exam now showed crescentic, haptic-shaped iris transillumination defects nasally and temporally (Fig. 1) and pseudophacodonesis. Ultrasound biomicroscopy (UBM) revealed a normal iris configuration, a single-piece IOL in the capsular bag, and a Soemmering ring (Fig. 2). No vitreous hemorrhage was noted.
We strongly suspected uveitis-glaucoma-hyphema (UGH) syndrome at this point. We discussed the options of ongoing observation versus surgery to remove the IOL. The decision was made to monitor the condition, with consideration of surgery if significant or recurrent hemorrhages or IOP elevation were noted. IOP-lowering medications and topical steroids were continued.
 |
|
IRIS TRANSILLUMINATION. Slit-lamp exam 1 month after presentation reveals crescent-shaped iris transillumination defects nasally and temporally (arrow). We also observed pseudophacodonesis.
|
Making the Diagnosis
Eight weeks after initial presentation, Ms. Ivanov returned acutely for decreased vision. In the right eye, her visual acuity was hand motions, and IOP was 32 mm Hg despite good compliance with IOP-lowering drops. A new microhyphema and diffuse vitreous hemorrhage were present, and the pseudophacodonesis appeared more dramatic than at the last examination, confirming the diagnosis of UGH syndrome in the setting of XFS and late zonular weakness.
 |
|
UBM. Ultrasound biomicroscopy 4 weeks after initial presentation shows single-piece IOL in the capsular bag and a Soemmering ring (arrow). There was suspicion that a haptic was abutting the ciliary body nasally, but it was poorly visualized by UBM.
|
Discussion
UGH syndrome was initially described in 1978 as a complication of first-generation anterior chamber IOLs that caused chafing of intraocular structures.1 It is now known that virtually any IOL can cause UGH syndrome, including modern anterior chamber IOLs, sulcus IOLs (both 3-piece and single-piece IOLs, which may be inadvertently or purposely placed in the ciliary sulcus2), and IOLs placed in the capsular bag.3,4
Blood-aqueous barrier compromise due to the chafing results in the classic triad of uveitis, glaucoma, and hyphema. IOP elevation can be caused by inflammation and resultant scarring of the trabecular meshwork, by pigment and hyphema clogging the meshwork, and by direct injury to the aqueous drainage complex. Iris transillumination defects, secondary neovascularization of the iris, and cystoid macular edema may also occur. The term “UGH Plus syndrome” has been used to describe the condition of patients such Ms. Ivanov, who, in addition, have vitreous hemorrhage.5
In-the-bag UGH. Single-piece IOLs in the capsular bag are a rare but increasing cause of UGH syndrome.4 Zhang et al. reported a patient with XFS and UGH syndrome 3 years after uneventful placement of a single-piece IOL in the capsular bag. They posit that the haptic-capsule complex chafed the posterior iris in the setting of subclinical pseudophacodonesis.4
Bryant et al. observed extensive fibrosis (Soemmering ring) around the haptics of the single-piece IOL in the capsular bag in a patient with UGH syndrome. They suspected that the fibrosis caused the IOL to tilt out of the iris plane, leading to haptic-iris and haptic–ciliary body chafing.3
Single-piece IOLs are now the most commonly implanted lenses in the United States. Given the aging of the U.S. population, ophthalmologists may encounter a growing number of cases of in-the-bag UGH syndrome in the future.
Treatment. Topical therapy with steroids and IOP-lowering medication, with or without cycloplegia, is the first-line treatment. Photocoagulation to ablate leaking vessels, intravitreal anti–vascular endothelial growth factor medications, and placement of a capsular tension ring to adjust IOL positioning have all been described as therapies for UGH syndrome.5 However, removal of the IOL is often required, as in our patient’s case. In a series of 109 eyes at a tertiary referral center, UGH syndrome was among the most common reasons for IOL exchange, accounting for 11.9% of cases.6
Key considerations. The presence of seemingly spontaneous hyphema along with elevated IOP and inflammation should prompt clinicians to consider UGH syndrome, even if the patient has a single-piece IOL in the capsular bag. This consideration is particularly important in eyes that are susceptible to zonular weakness, such as those with XFS or a history of ocular trauma. Careful slit-lamp examination, gonioscopy, and UBM are important tools in making the diagnosis of UGH syndrome.
Our Patient
Ms. Ivanov underwent pars plana vitrectomy, IOL removal, and placement of a scleral-sutured Akreos AO60 IOL. At 4 months following surgery, best-corrected visual acuity was 20/25 in the right eye, and IOP was in the midteens on a prostaglandin analogue and fixed-dose combination drops. Serial OCT and visual field testing were stable. No further episodes of decreased vision were reported.
___________________________
* Patient name is fictitious.
___________________________
1 Ellingson FT. J Am Intraocul Implant Soc. 1978;4(2):50-53.
2 Chang DF et al. J Cataract Refract Surg. 2009;35(8):1445-1458.
3 Bryant TK et al. J Cataract Refract Surg. 2017;43(7):985-987.
4 Zhang L et al. J Cataract Refract Surg. 2014;40(3):490-492.
5 Sousa DC et al. J Curr Glaucoma Pract. 2016;10(2):76-78.
6 Davies EC, Pineda R. J Cataract Refract Surg. 2016;42(9):1262-1267.
___________________________
Mr. Woodward is a third-year medical student at Weill Cornell Medical College. Dr. Mahrous is a third-year ophthalmology resident at Weill Cornell/New York Presbyterian Hospital. Dr. Gupta is a vitreoretinal specialist, and Dr. Van Tassel is a glaucoma specialist; both are at Weill Cornell Medicine. All authors are in New York City. Financial disclosures: None.