Download PDF
Seven years after an outbreak of fungal endophthalmitis from contaminated triamcinolone, ophthalmologists whose patients were infected have reached some sobering conclusions about the difficulties of treating such cases.
In 2012, 30 eyes nationwide were infected with a plant fungus, Bipolaris hawaiiensis, from intravitreal injections of contaminated triamcinolone.1,2 The drug had been compounded by the now-defunct Franck’s Compounding Pharmacy in Ocala, Florida.
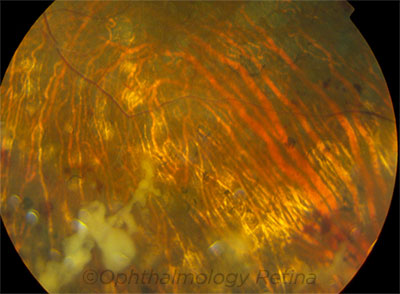 |
PRESENTATION. This patient’s visual acuity was 20/50 at onset of fungal endophthalmitis. He eventually underwent enucleation.
|
Five-year outcomes. Ophthalmologists who treated 23 of these patients (25 eyes) in Los Angeles and New York City have now reported their five-year follow-up outcomes.3
Treatment challenges. The outbreak started as an acute crisis but evolved into a chronic, puzzling management problem, the retrospective chart review revealed. For example:
- Some infections presented as late as 10 months postinjection.
- Despite appearing sterile after treatment, eyes that were enucleated still had hyphae present. (Because the organism is difficult to culture, the hyphae’s viability could not be determined, the authors reported.)
- Intravitreal antifungal injections, vitreous tap, and pars plana vitrectomy did not resolve the infections. Nor did a standard, on-label systemic regimen for treating fungal infections (200 mg oral voriconazole twice a day for six weeks). “With all the patients, as soon as the oral voriconazole was stopped after six weeks, the infection came back with a vengeance,” said lead author Kent W. Small, MD, who practices in Los Angeles and Glendale, California.
- Patients required prolonged systemic off-label, high-dose treatment with oral voriconazole (300 mg twice daily for six to 12 months) to eliminate clinical signs of infection.
- Despite apparently successful treatment, some of the eyes continued to deteriorate, most frequently from hypotony. Only eight of the 25 eyes had final visual acuity (VA) of 20/50 or better. Five eyes had to be enucleated, and the VA in an additional five eyes was light perception or no light perception.
Need for prompt communication. In addition to such clinical lessons, the endophthalmitis outbreak was an example of the importance of the need for meticulous oversight of compounding (and other) pharmacies as well as the importance of professional transparency among ophthalmologists when an outbreak occurs, Dr. Small said.
In Dr. Small’s own practice, 17 eyes were infected with B. hawaiiensis. “No practice—private, academic, or governmental—is immune to receiving contaminated medication from any pharmacy. But when this sort of incident happens, a feeling of isolation is overwhelming because you realize you are on your own in unchartered waters,” Dr. Small said. After his initial feelings of dismay and embarassment, he said, “I soon realized I did nothing wrong. There is nothing I could have done differently to have prevented this.”
He concluded, “The ophthalmic community needs to know about these kinds of incidents. We need to alert each other and learn from each other how to handle them.”
—Linda Roach
___________________________
1 Mikosz CA et al., and the Fungal Endophthalmitis Outbreak Response Team. Emerg Infect Dis. 2014;20(2):248-256.
2 MMWR Morb Mortal Wkly Rep. 2012;61(17):310-311.
3 Small KW et al. Ophthalmol Retina. 2019;3(2):133-139.
___________________________
Relevant financial disclosures—Dr. Small: None
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Asaoka Kowa: S; Oculus: S; Reichert Technologies: S.
Mr. Boyle None.
Dr. Hsu Genentech/Roche: S; Ophthotech: S; Santen: S.
Dr. Palanker DigiSight: C; Johnson & Johnson: C; Pixium Vision: C,P; Topcon Medical Systems: C,P.
Dr. Small None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review