Download PDF
Researchers at Mayo Clinic have harnessed a common imaging technology in a way that may be a game-changer in assessing Fuchs endothelial corneal dystrophy (FECD).1 Using Scheimpflug imaging, they found that the presence of three parameters—loss of regular isopachs, displacement of the thinnest point of the cornea, and focal posterior surface depression—could be used to predict risk of disease progression or need for surgical intervention.
And although central corneal thickness (CCT) is often used to gauge whether keratoplasty is needed, CCT proved to be a weak predictor of prognosis, especially in the absence of previous CCT measurements and clinically definite edema. Another prognostic factor—anterior corneal backscatter—also proved to be weakly predictive.
“Clinicians should recognize that Scheimpflug tomography can determine if corneas with FECD might be contributing to visual symptoms, especially in the absence of an advanced cataract, other comorbidity, or clinically obvious edema at the slit lamp,” said Sanjay V. Patel, MD, FRCOphth, at the Mayo Clinic in Rochester, Minnesota. “The images are easy to acquire and interpret in clinical practice, and they help determine prognosis.”
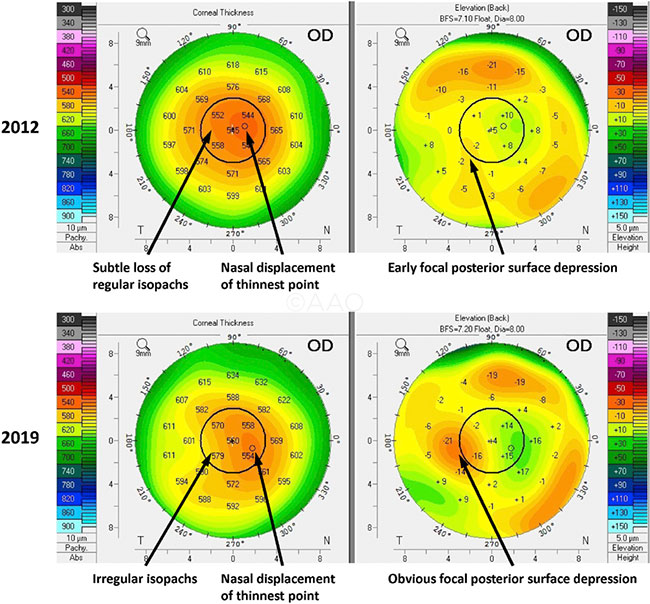 |
FUCHS PROGRESSION. (Left column) Pachymetry maps were evaluated for loss of parallel or almost circular isopachs and for displacement of the thinnest point of the cornea (small circle). (Right column) Posterior elevation maps were evaluated for focal posterior surface depression. All images taken in the same eye.
|
Study specifics. For this study, Dr. Patel and his colleagues used the Pentacam HR (Oculus) to evaluate 56 patients (96 eyes) who had a range of FECD severity. The patients were followed for a median of five years to determine whether imaging findings, anterior corneal backscatter, or CCT could predict disease prognosis. Progression was defined as the new onset of clinically definite edema, a 5% or more increase in CCT, or surgical intervention with endothelial keratoplasty (EK).
Evaluating risk. At five years, when none of the three Scheimpflug parameters were present, the cumulative risk of progression was 7%, reflecting the natural progression of the disease. This increased to 48% when one or two parameters were present and to 89% when all three parameters were present.
Loss of regular isopachs and displacement of the thinnest point of the cornea were independent and clinically important risk factors for progression, conferring an 8-and 4-fold risk of progression, respectively. In contrast, anterior corneal backscatter was a weak predictor of FECD progression/intervention, and CCT was a risk factor only in eyes with definite edema at slit-lamp exam.
Risk after cataract surgery. Researchers also considered the 27 eyes that underwent uncomplicated cataract surgery after enrollment. The four-year cumulative risk of progression/intervention was 0% when no tomographic parameters were present, 50% with one or two parameters, and 75% with all three parameters.
Who should be imaged? Patients who have clinically obvious edema at the slit lamp do not need Scheimpflug imaging, Dr. Patel said. Neither do patients who do not appear to have edema and are asymptomatic with normal vision. “For other patients with FECD, or when cataract surgery is being considered, we strongly recommend imaging.”
He suggested that clinicians might use the results to counsel patients about whether EK may be appropriate. He added that the study’s findings might prevent unnecessary EK procedures, including those undertaken too early in the course of the disease.
A note on CCT cutoffs. “There are published articles that suggest proceeding to keratoplasty above different CCT cutoffs,” Dr. Patel. “I hope that these notions can be abandoned given our findings that CCT was very weakly predictive of prognosis, if at all, whereas the tomographic features were highly predictive.”
—Miriam Karmel
___________________________
1 Patel SV et al. Ophthalmology. Published online Sept. 27, 2019.
___________________________
Relevant financial disclosures—Dr. Patel: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Choritz Nonfinancial support for other clinical studies from Allergan, Novartis, and Santen.
Dr. Jonker None.
Dr. Patel None.
Dr. Seitzman Dompé: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review