By Arthur Stone, Contributing Writer, interviewing Lynn K. Gordon, MD, PhD, Andrew G. Lee, MD, Ronald W. Pelton, MD, PhD, and Alfredo A. Sadun, MD, PhD
Download PDF
Giant cell arteritis (GCA) may be relatively rare, but it’s a diagnosis that you don’t want to miss. “The short window for diagnosis and treatment and the risk of severe bilateral loss of vision make the high stakes of this condition clear,” said Ronald W. Pelton, MD, PhD, who practices in Colorado Springs, Colorado.
In the United States, the lifetime risk of developing GCA is estimated at 1% in women and 0.5% in men, with incidence increasing with age.1 And the disease can have significant visual and systemic consequences, including blindness, myocardial infarction, and stroke. But after many years in which corticosteroids were the only treatment option, tocilizumab (Actemra) was approved for GCA in 2017, and a number of others are under investigation.2
 |
|
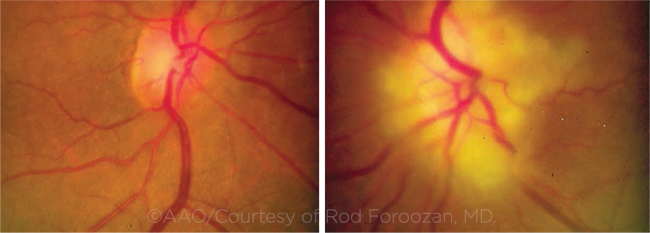
PRESENTATION. In this case of GCA, the patient’s left optic disc is pallid and swollen. A massive infarction, with extension into the surrounding retina, is evident. In contrast, the right optic disc is normal, with a cup-to-disc ratio of about 0.3.
|
Diagnostic Basics
The most-easily-recognized ocular sign of GCA is loss of vision; diplopia and eye pain also may occur. The classic systemic symptoms include new-onset headache, scalp tenderness, jaw claudication, fever, fatigue, and weight loss.A previous diagnosis of polymyalgia rheumatica should suggest GCA due to the similarities of systemic inflammation between the two conditions.
However, the disease is notorious for its range of clinical manifestations. “Just when I think I know the presentation, GCA has a new wrinkle in store for me,” said Alfredo A. Sadun, MD, at the Doheny Eye Institute in Los Angeles.
Exam considerations include the following:
Ophthalmic exam. A full ophthalmic examination should be performed in all cases of suspected GCA, including funduscopy, slit-lamp biomicroscopy, color vision evaluation, and visual acuity and visual fields in both eyes. Even apparently unaffected eyes may exhibit subtle defects suggesting a possible risk for further loss of vision in that eye. In addition, the temporal artery should be evaluated (by palpation) for tenderness and pulsation.
Patient history and lab tests. A careful patient history is key, particularly as some manifestations of the disease can be mild or transient in nature, and not all patients experience all symptoms. For instance, with “occult GCA” the patient may have ocular signs and symptoms but no systemic issues.
Blood tests should be ordered for C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete blood count (CBC), and platelet counts. A temporal artery biopsy (TAB) is considered the gold standard for diagnosis.3
Subtypes of GCA. There appear to be four distinct subtypes of GCA, which adds to the diagnostic challenge:4
- cranial arteritis with severe ischemic complications (including visual loss and cerebral ischemia)
- large vessel arteritis
- aortitis leading to aortic dissection, aneurysm, and aortic rupture
- a systemic inflammatory syndrome with nonstenosing vasculitis and “isolated” polymyalgia rheumatica
At this time, these subtypes can be identified only by clinical and radiologic findings. However, as research on GCA-related biomarkers progresses, it may be that each of these subtypes will require a targeted treatment regimen, said Andrew G. Lee, MD, at the Blanton Eye Institute in Houston.
Treatment Update: Tocilizumab
Corticosteroids have been used to treat GCA for decades, and they have been shown to reduce the risk of vision loss in the fellow eye. However, while some patients with GCA respond well to corticosteroids and can be tapered off of treatment relatively quickly without complication, others require long-term treatment, which carries a significant risk of steroid side effects.4
New steroid-sparing therapies—notably tocilizumab (TCZ)—have shown promise in causing disease remission and reducing cumulative steroid dose.4 But should TCZ be routinely used to treat the condition?
Need to tread carefully. TCZ, which blocks the interleukin-6 pathway, is also used to treat rheumatoid arthritis and juvenile idiopathic arthritis. Although TCZ is “revolutionary in that it was the first steroid-sparing agent that was successful in GCA, multiple questions remain about the clinical settings in which TCZ should be used,” said Lynn K. Gordon MD, PhD, at the University of California, Los Angeles. These include dose (amount, frequency, and length of treatment), concomitant systemic steroid dose, and tapering of steroids while a patient is on the medication.4
Risk-benefit calculation. Dr. Gordon is of the opinion that TCZ should not universally be used to treat GCA. Instead, she said, traditional systemic steroid therapy alone should be the first-line agent used for a patient who presents with neuro-ophthalmic symptoms of GCA if the patient is able to comply with and tolerate the therapy.
She outlined the potential adverse effects of TCZ, including neutropenia, which occurs among 4% of patients.4 In addition, the treatment schedule—which involves weekly or alternate-week injection—can be challenging for elderly patients, she noted.
“It is a little early to completely appreciate the long-term complications with TCZ treatment,” Dr. Sadun agreed. He also cited the drug’s cost. However, he said, “for the treatment of GCA, TCZ is a game changer”—particularly as it enables clinicians to reduce a patient’s cumulative dose of prednisone.
Tailored treatment. “Patients with GCA who quickly respond to steroids, who do not develop significant steroid-associated adverse effects, and who are able to taper steroids to a level with an acceptable safety profile likely do not require TCZ,” Dr. Gordon said.
She also noted that some patients on TCZ experience recurrence of GCA, which can lead to severe and irreversible morbidity, including permanent vision loss. “The risks of long-term therapy with TCZ are unknown. I recommend proceeding with caution and using TCZ in patients in whom it is difficult to reduce corticosteroids or [if] there are contraindications for long-term high-dose corticosteroid use. Perhaps after additional experience with TCZ, it may become a first-line therapy.”
Pathogenesis of GCA
Although the pathogenesis of GCA is not entirely understood, it may occur because of genetic variations in the major histocompatibility complex region in specific human leukocyte antigen (HLA) alleles. Other non-HLA genes associated with GCA encode key proteins involved in T-cell function, as well as genes encoding interleukins and cytokines. Epigenetic modifications, namely hypomethylation of proinflammatory genes, have been shown to initiate onset of GCA.1
The pathogenesis of GCA also involves unknown environmental factors that activate dendritic cells in the adventitia of arteries. This activation sets off a cascade of events that eventually leads to vascular occlusion and the ischemic symptoms of GCA.1
___________________________
1 Samson M et al. Autoimmun Rev. 2017;16(8):833-844.
|
Predicting Response to Tx
To date, there are few quantitative markers that can predict disease severity or prognosis in GCA.3 Surrogates for disease severity have included visual acuity, progressive loss of vision, and the number of relapses during the time a patient is tapered off of steroids.
But a study by Dr. Lee and his colleagues found a specific marker: the CD68+ macrophage marker found on temporal artery biopsy specimens.3
The researchers used CD68 immunohistochemistry to label macrophages in the TABs, and they measured the association between CD68+ cells per histologic section and patients’ response to treatment. They found that, of 42 patients who underwent unilateral TAB for GCA, those who were refractory to initial steroid tapers and those who were eventually placed on immunomodulatory therapy had a greater number of CD68 cells per section.
Dr. Lee and his coauthors encouraged further study of the use of quantitative immunologic markers with TAB specimens. This might enable clinicians to identify which patients are at increased risk of developing severe disease and might benefit from earlier and more aggressive treatment for GCA, they wrote.
___________________________
NEXT MONTH GCA, Part 2: Malpractice claims and three case studies.
___________________________
1 Fein AS, Ko MW. Semin Neurol. 2019;39(6):673-681.
2 www.clinicaltrials.gov. Accessed Aug. 13, 2020.
3 Sultan H et al. Am J Ophthalmol. 2018;193:45-53.
4 Sadun A, Gordon L. J Neuroophthalmol. 2020;40(1):117-121.
___________________________
Dr. Gordon is senior associate dean in the Office of Equity and Diversity Inclusion and professor of ophthalmology at the David Geffen School of Medicine at the University of California, Los Angeles. Relevant financial disclosures: None.
Dr. Lee is chair of ophthalmology at the Blanton Eye Institute, Houston Methodist Hospital. Relevant financial disclosures: None.
Dr. Pelton practices oculofacial plastic surgery in Colorado Springs, Colo. He is a board member of OMIC and an Academy Trustee-at-Large. Relevant financial disclosures: OMIC: C.
Dr. Sadun is the Flora L. Thornton Endowed Chair at Doheny Eye Centers–UCLA and vice chair of ophthalmology at the University of California, Los Angeles. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Gordon None.
Dr. Lee Horizon: C.
Dr. Pelton OMIC: C.
Dr. Sadun GenSight: S; Santhera: S; Stealth BioTherapeutics: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|