This article is from February 2004 and may contain outdated material.
When a second gene that causes adult onset primary open-angle glaucoma was discovered, the researcher who found it hailed it as a medical breakthrough. “Decades before even the first signs of the disease have occurred, we will know who is at risk and can take preventive action,” Mansoor Sarfarazi, PhD, said in a February 2002 news release issued by the University of Connecticut Health Center, where he is director of the Molecular Ophthalmic Genetics Laboratory and professor of human genetics.
Two years later, however, the most likely preventive action is more aggressive observation of at-risk patients.
It’s true that an individual with a mutation in that gene—which is on chromosome 10p14 and was named Optineurin, for “Optic Neuropathy Inducing” protein—has a greater risk of going on to develop POAG. But no new treatments or medications accompanied the discovery of Optineurin or any of the other genes associated with glaucoma. “We do not have diagnostics that are helpful to the average glaucoma patient,” said Wallace L. M. Alward, MD, professor of ophthalmology and director of the Glaucoma Service at the University of Iowa. “We don’t have gene-specific therapy. For right now, for the average glaucoma patient, there’s no benefit in genetic testing.”
 |
|
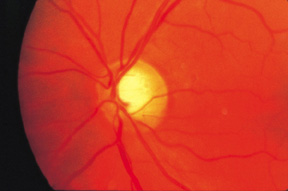
Patients with OPTN mutations often have vertical optic cup elongation. Credit: BCSC, Section 10 / Elizabeth A. Hodapp, MD.
|
To Market, to Market
Nonetheless, a test for the Optineurin gene is being developed by InSite Vision. This is the same company that has marketed a test for the myocilin gene (they call it TIGR), which is associated with POAG, and has developed a test for the CYP1B1 gene, which is associated with congenital glaucoma.
If you order a genetic test for a patient, what will you learn—and will that knowledge affect the course of treatment? Unlike tests for diseases such as Huntington’s, the glaucoma tests are not 100 percent predictive. Yet they will indicate the presence or absence of the gene mutation associated with several forms of glaucoma. If a mutation is present in asymptomatic individuals, you know that they are at extremely high risk for developing the disease.
In POAG, which is an autosomal dominant condition, 50 percent of the children will inherit the disease and the other 50 percent will not, Dr. Sarfarazi explained. Presence of a gene mutation ratchets that risk up to 90 percent or more—or down to 10 percent or less, he said. “Basically, the discovery of every new gene of glaucoma will provide more tools for being able to do this presymptomatic diagnosis,” he said, adding, “This is not a clinical diagnosis. It is a molecular diagnosis.”
Either way, that diagnosis won’t radically alter the course of treatment. “Probably the best thing is to monitor [patients with the mutation] on a regular basis,” Dr. Sarfarazi said.
“I rarely order a genetics test, even though I can do it for free,” said Dr. Alward, whose lab is investigating a drug that might interrupt the production of myocilin in cell culture.1
However, it would be reasonable to consider testing for myocilin mutations in families with a very strong history of early onset glaucoma, Dr. Alward added. The first step would be to test the patient who has juvenile open-angle glaucoma. If that patient has a mutation in the myocilin gene, it might be appropriate to test the other children in the family, to see if they have the same mutation. “If they do,” said Dr. Alward, “we watch them like a hawk.” On the other hand, if they don’t have the mutation, “we don’t have to worry about them.”
Similarly, Dr. Alward might test for rare diseases such as aniridia or Rieger’s syndrome, when a patient has features of the disease, but diagnosis is elusive.
But in most cases, as Richard K. Parrish II, MD, notes in Archives of Ophthalmology, the diagnosis of glaucoma should remain a clinical, rather than molecular, enterprise.2 “At present, the most effective method of detecting damage is by optic nerve examination or screening visual field testing,” he writes. “Genetic investigation should remain a research activity until we develop tests that are highly sensitive and specific and until we really know what to do with the information gathered.”
“I would agree 100 percent with Dr. Parrish,” said Dr. Alward. “We’re very excited about molecular genetics of glaucoma, and eventually research will open a lot of doors in terms of diagnosis and treatment. But with rare exceptions, we’re not there yet.”
Dr. Sarfarazi’s lab, for example, is studying the biology of the Optineurin protein (not to be confused with the gene), to see how it functions to produce glaucoma. “We want to understand how this protein functions at the normal level first,” he said. Then it will be possible to understand the difference between normal and abnormal expression.
However, until a genetic cure is discovered, it’s still business as usual at the clinical level. As Dr. Parrish points out in his Archives editorial, “As an intern I was cautioned against ordering laboratory tests unless I would use the results to change my management plan. This advice still seems prudent today.”
______________________________
1 The University of Iowa’s Carver Laboratory is developing tests for several glaucoma genes. Seewww.carverlab.org, call 866-844-2522 or e-mail carverlab@molpc.ophth.uiowa.edu.
2 Arch Ophthalmol 2002;120(9):1204–1205.
______________________________
Dr. Alward coholds several patents on genetic discoveries but has no financial interest in his research. Dr. Sarfarazi does not have a personal financial interest in his research; however, the University of Connecticut has a licensing agreement with InSite Vision.
The ABCs of GLC
Glaucoma is a genetically heterogeneous condition, for which only a few genes have been discovered. Myocilin mutations, which cause 3 to 5 percent of POAG, are “a very small contributor” to that disease, Dr. Alward said. Optineurin mutations are much rarer, causing less than 0.1 percent of glaucoma in a group of 1,048 patients described by Dr. Alward and colleagues.1 “We have no idea how many genes are out there,” he added. “If there were a ‘big fish’ gene out there, it probably would have been found by now.”
To date, three gene mutations have been identified for primary forms of glaucoma:
- Mutations in the CYP1B1 (or GLC3A) gene have been identified in approximately 85 percent of the families with primary congenital glaucoma. They also have been found in 33 percent of the isolated cases with no prior family history.
- Mutations in myocilin (also called TIGR, or GLC1A) cause almost all familial juvenile-onset POAG and 3 to 5 percent of adult-onset POAG.
- Mutations in Optineurin (OPTN or GLC1E) cause autosomal dominant normal-tension glaucoma.
Genes for rare conditions that have been identified include:
- Mutations in the PITX2 and FOXC1 genes, which can cause Rieger’s syndrome.
- Mutations in PAX6, which can cause aniridia.
The CYP1B1 gene is also known as GLC3A, a name that refers to its locus, or location on the genetic map. The locus names for various forms of primary glaucoma all begin with GLC, the abbreviation for glaucoma given by the Human Genome Organization nomenclature committee.
Numbers following GLC indicate different forms of the disease:
- GLC1 represents various primary open-angle glaucomas. Within GLC1, there are genes at five loci, A-F. But only genes at loci A and E have been identified. The genes at loci B, C, D and F have not yet been discovered.
- GLC2 represents closed-angle glaucoma genes. None has been found yet.
- GLC3 represents congenital glaucomas. Three genetic loci (GLC3A to GLC3C) have been identified for this condition, but only one gene, CYP1B1/GLC3A, has been found.
______________________________
1 Am J Ophthalmol 2003;136(5): 904–910.
|
All in the Family
Physicians on the front lines are needed to identify the families that are the mainstay of genetic research.
“What fuels these kinds of studies are families,” Dr. Alward said. “Any lab that’s interested in molecular genetics is always on the lookout for a great family”—that is, one with many living, affected members with any kind of glaucoma. He sets the lower limit at 15.
Dr. Sarfarazi screened 54 families for his research that led to the discovery of the Optineurin gene. Each of the families has more than two affected individuals, and one family has 18 affected individuals.
If you’ve encountered a “great family,” report it to one of the centers engaged in genetic research. There is no central clearinghouse for such reporting. Dr. Alward suggests reviewing the literature to see which labs have studied the specific disease that your family represents.
|