Download PDF
Although intraocular pressure is a common culprit in primary open-angle glaucoma, several other mechanisms are being explored. An in-depth look at the role that cerebrospinal fluid pressure may play in this disease.
Cerebrospinal fluid (CSF), the normally clear, watery fluid that cushions the brain and spine, has long been the domain of neurologists. Lately, CSF and the pressure it exerts on the lamina cribrosa has attracted the attention of ophthalmologists who have been looking beyond intraocular pressure (IOP) to explain the pathophysiology of glaucoma.
“IOP doesn’t happen in isolation,” said John P. Berdahl, MD, at Thompson Vision, Sioux Falls, S.D. He regards glaucoma as a 2-pressure disease. “On the other side of the optic nerve is the CSF,” he said. “I don’t care if the eye pressure is high or low. I care what it is in relation to CSF pressure [CSFp].”
That there are multiple mechanisms for glaucoma is not a new concept. “CSFp is the new kid on the block as one of the mechanisms that may affect the pathophysiology of glaucoma,” said Malik Y. Kahook, MD, at the University of Colorado School of Medicine. Other mechanisms explored by researchers include episcleral venous pressure, blood pressure, corneal hysteresis, and ocular perfusion pressure.
“The answer will probably lie in all of them,” said David Fleischman, MD, at the University of North Carolina at Chapel Hill. “We haven’t been satisfied with the idea that IOP alone explains glaucoma.”
CSFp was recognized as a potential contributor to glaucoma in 1908 by K.I. Noishevsky. The Russian ophthalmologist was the first to hypothesize and test, in a dog, the idea that CSFp is an important factor in the development of glaucomatous optic neuropathy. It wasn’t until the 1970s, however, that the idea started to gain traction among several groups of investigators in the United States and abroad. The data they’ve been amassing support the hypothesis that CSFp is lower in patients with primary open-angle glaucoma (POAG) than in patients without glaucoma. Findings indicate CSFp is even lower in patients with normal-tension glaucoma (NTG). Also, CSFp appears to be higher in patients with ocular hypertension (OHT) than in those with normal IOP.
But the clinical application of this knowledge lags behind, since the only proven and accurate way to measure CSFp is by lumbar puncture or with an intraventricular probe. Even then, what do you do with that information?
Still, researchers are confident that the interplay of pressures on either side of the lamina cribrosa—a delicate balance between CSFp and IOP—may help to explain the disease process.
The Studies
Dr. Berdahl pointed to 2 studies that support the role of CSFp in glaucoma.
In one study, Dr. Berdahl and colleagues found that mean CSFp was 33% lower in subjects with POAG compared with nonglaucomatous controls (9.2 mm Hg compared with 13.0 mm Hg).1 This suggests that translaminar pressure difference is influenced by both IOP and CSFp, he said.
These findings were corroborated in a prospective study conducted in Chinese patients with POAG who were compared with nonglaucomatous controls.2 Patients with high-pressure POAG had lower CSFp compared with the controls, while NTG patients had even lower CSFp than the other 2 groups.
CSFp and age, BMI, and race. The research linking CSFp to glaucoma prompted Dr. Fleischman and colleagues to apply other risk factors for glaucoma to the CSFp construct.3 They asked what relation, if any, CSFp has to other possible risk factors, such as increased age, BMI, and race. “I wanted to simulate a normal population and see if we could trend CSFp across these different variables,” he explained. The researchers reviewed the electronic medical records of all patients who underwent lumbar puncture at the Mayo Clinic from 1996 to 2009.
The age findings were most robust. CSFp was fairly stable for the first 5 decades and then declined steadily after age 50. The observed change in CSFp parallels the rise in prevalence of POAG as people age. This suggests that either CSF production decreases or CSF outflow resistance diminishes with age, Dr. Fleischman explained. While CSFp declined with age, BMI was positively associated with CSFp in every age group studied. A lack of statistical power precluded finding an association with race. (The Mayo Clinic population is predominantly Caucasian.)
Balance Goggles: A Space Age Connection
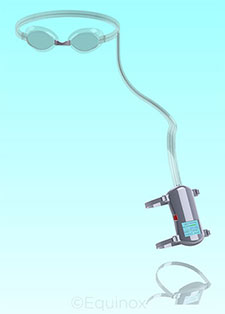
FAR OUT. Technology designed for use in specs may find its way to glaucoma patients.
|
There are 2 sides of CSFp-IOP imbalance. Low CSFp appears to play a role in the pathogenesis of glaucoma. But elevated CSFp is associated with papilledema, a problem astronauts experience from long-term exposure to microgravity. Either way, the optic nerve can suffer damage.
The federally funded National Space Biomedical Research Institute Industry Forum, which has a particular interest in the papilledema side of the equation, has offered research grants to those who are trying to find ways to prevent vision problems in space. One of those grants went to Dr. Berdahl, who subsequently developed pressurized goggles to balance IOP with elevated CSFp. While his Balance Goggles (Equinox) were developed with astronauts in mind, the spin-off technology could represent a nonsurgical, nonpharmaceutical treatment for glaucoma, Dr. Berdahl said.
The prototype Balance Goggles look like ordinary swim goggles, with a tube attached to a vacuum pump that runs from one side. The goal is to balance IOP and CSFp, which is done with a dial on the pump that controls the pressure inside the goggles to within 1 mm Hg.
Balance Goggles would be worn for a specific amount of time—probably while the wearer is asleep—to alleviate the pressure differential that may be causing the visual problems experienced by both astronauts and glaucoma patients.
|
How Does It Work?
“The CSFp hypothesis isn’t that hard to understand,” said R. Rand Allingham, MD, at Duke University. “It’s a pressure difference. That’s all we’re talking about.”
The difference is between IOP on one side of the lamina cribrosa and CSFp on the other. If CSFp is lower than IOP, the pressure inside the eye will generate a force that can cause the optic nerve to bow backward and cup. In the opposite situation, when CSFp is higher than IOP, as in pseudotumor cerebri, the optic nerve can swell, producing papilledema.
To explain the biomechanics, Dr. Allingham uses a teeter-totter analogy. At one end, or anteriorly, is the intraocular space. At the other end, or posteriorly, is the subarachnoid space.
“All we’re saying is that nerve fibers that form the optic nerve have to go from the eye pressure world to the brain pressure world. The point where those pressures meet is the lamina cribrosa. That’s the fulcrum. Those axons tolerate only a specific range of difference before they stop functioning normally. If it exceeds the tolerated balance, the function of the fibers is impaired. If impaired long enough, the retinal ganglion cells will die. We know that’s where the damage is,” Dr. Allingham said. “So whenever one end of the teeter-totter gets too high or too low, you end up with a glaucoma problem” or papilledema, depending on the direction of the pressure differential.
In other words, the optic nerve is exposed to 2 independent pressurized regions that are separated by the lamina cribrosa. The pressure difference across the lamina cribrosa—the translaminar pressure difference—is an important number, Dr. Allingham said. “We feel that measuring the pressure differential is a key issue in managing glaucoma.”
But currently, we have a problem, he added. “We’re controlling only one end of the teeter-totter because we don’t know the number on the other end.” So if, for example, you reduce the IOP to what you think is an acceptable range, but the patient has a very low CSFp, the IOP may not be low enough for that individual. On the other hand, if the patient’s CSFp is higher than normal, you may be targeting a lower IOP than necessary and overtreating.
The black box. “Doctors have been using IOP for years. Now it turns out the pressure directly behind the eye is probably important,” Dr. Fleischman said. “The biggest black box is the orbital space—the perioptic subarachnoid space. We always talk about the translaminar pressure gradient. The problem is, we don’t have the pressure behind the orbit; we have the number at the spine.” And the pressure at the level of the lumbar spine may not be the same as the pressure behind the eye, for example, when a person is standing. Since the CSF is a fluid column, the pressure behind the eye would be much lower than that at the base of the spine, said Dr. Fleischman, who has been studying fluid dynamics to better understand how fluid circulates between the brain and the orbit. “There hasn’t been anything that has shown us that the pressure in the orbit is close to what we measure in the spine.”
Measuring CSFp. “We need more information on how CSFp changes in habitual positions—recumbent, sitting down, standing,” agreed Dr. Kahook. “We know that IOP is elevated when lying down. We also know that CSFp is variable depending on position. So if pressure is dynamic, are we oversimplifying the relationship between IOP and CSFp?” As research into this topic continues, Dr. Kahook said that he would like to see a study monitoring CSFp in a sleep lab that could answer some questions about pressure dynamics.
Assessing the subarachnoid space. Much of Dr. Allingham’s own research has relied on retrospective CSFp data acquired by lumbar puncture performed for reasons unrelated to ophthalmic conditions. But some studies have used magnetic resonance imaging (MRI) to estimate either increased or decreased intracranial pressure.
In one study, researchers used MRI to test the hypothesis that in patients with POAG, the optic nerve subarachnoid space width may be narrow due to lower CSFp.4 They argue that the best way to measure this tiny space may be with MRI, which offers high resolution of the soft tissue, as well as good imaging of the CSF and of the whole length of the optic nerve in the orbit. Using MRI, they measured 39 glaucoma patients in a supine position and found that the orbital optic nerve subarachnoid space is significantly narrower in POAG patients with normal IOP than in those with high IOP or in the 21 healthy controls. The study is important, Dr. Allingham said, because it supports the hypothesis that the CSFp is lower in many individuals with glaucoma than in those who don’t have glaucoma. But these results are drawn by inference because the study, like all others, could not directly measure CSFp behind the eye.
Other measurements. Similarly, OCT is not a direct measure of CSFp, though recent technical improvements such as enhanced depth imaging (EDI) help visualize pores, axon bundles, and the insertion site and thickness of the lamina cribrosa.
Dr. Berdahl is most intrigued by an intracranial pressure (ICP) monitor developed by Vittamed (Boston). The noninvasive device uses dual Doppler ultrasound to measure both intracranial blood flow and orbital outflow within the ophthalmic artery. Vittamed claims the technique has proved comparable to existing invasive measurement methodologies, including intraventricular catheters and lumbar puncture. After clinical trials in Europe, the company’s neuromonitor recently received the CE mark, the European approval for marketing. Only a few devices are in use in the United States, including one at NASA, Dr. Berdahl said.
 |
|
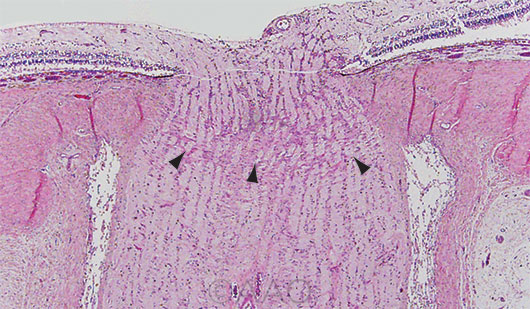
THE “FULCRUM.” Longitudinal section of the optic nerve with arrowheads pointing to lamina cribrosa.
|
Clinical Implications?
According to Dr. Fleischman, we won’t be incorporating the concept of CSFp clinically until we better understand CSF dynamics.
Dr. Kahook agreed that we need more information: “There are many retrospective studies that tie CSFp in with existing clinical data. This is a field of research with a growing body of knowledge, but it is still in its infancy. The key is taking it to the next level, collecting prospective data and correlating CSFp findings with disease progression metrics.”
Surrogate or direct measures? Dr. Kahook would like to find a way of moving beyond surrogate metrics to measure glaucoma, noting that Goldmann applanation tonometry is a surrogate for IOP, while OCT gives a general idea of retinal ganglion cell and axonal thickness but doesn’t directly measure apoptosis of cells.
Even if we’re not able to directly measure the pressure in the orbit, said Dr. Fleischman, there may be some algorithm that will allow estimation of pressure behind the eye. “Then we may have a breakthrough in understanding how the system works and be able to predict why some [people] get glaucoma and some do not.”
Such a breakthrough could help to explain why 20% to 30% of glaucoma patients have no history of elevated IOP, Dr. Berdahl said. (In some populations, that number is far higher. The LALES study, for example, found that 82% of Latino Americans with open-angle glaucoma had an IOP of 21 mm Hg or lower.5) Conversely, some patients with OHT don’t develop glaucoma and may be protected by higher CSFp, he said. “We’ve been missing a piece of the puzzle,” Dr. Berdahl said. “I believe that key is CSFp.”
Considering the role of CSFp. “Anytime I see a normal-pressure glaucoma patient who continues to get worse despite well-controlled pressures, I start to think maybe the CSFp is low,” Dr. Fleischman said. “But I wouldn’t start altering CSFp for a refractory glaucoma because I don’t know what it would do to the rest of the brain,” he added. Of his study of fluid dynamics, Dr. Fleischman said, “I’m not leading this project to change the CSFp. My interest is in understanding it.”
Dr. Berdahl envisions a time when we will have a noninvasive way to measure CSFp and use that information to set target eye pressures to prevent people going blind from glaucoma. “But we do not yet know how much risk is associated with a specific CSF pressure like we do for IOP.”
Dr. Berdahl spoke of 2 competing theories of the basic pathophysiology of glaucoma—axonal transport and ocular perfusion. Knowing which is at play could help further our understanding of CSFp and the role it might play in patient care, he said. Does a difference between IOP and CSFp inhibit blood flow or does it affect axonal transport? “I believe glaucoma is more of an axonal transport disease because glaucoma is a disease of years and decades, whereas most vascular conditions are quick,” he said, cautioning that we don’t yet have the science to support that concept. But if true, it suggests that glaucoma may be more of a metabolic disease than a vascular one, with the IOP-CSFp difference inhibiting axonal flow.
Early days. “Let’s say we find that CSFp is a player. What do we do with that?” Dr. Kahook said. “Can we reliably and safely influence CSFp? How do you do that? What’s the mechanism?” First, he said, we need to tie CSF with clinical metrics like OCT and visual field to see if all the pieces fit together.
“The CSFp concept is in an infantile state,” Dr. Fleischman said. “The more information we acquire, the more we can say it makes sense or not. The evidence so far is pointing toward a relationship between CSFp and glaucoma. It’s just the quantifiable nature that we’re having difficulty with.”
Dr. Allingham agreed. “The dynamics and function of CSFp in general are remarkably poorly understood. For such an important component of the body, it screams out for attention. This is one we’ve known about for a while now, but [we] still are missing enormous parts of it.” Having said that, he predicted we will gradually learn more. Then we may use CSFp along with variables such as central corneal thickness, family history, age, and genetics to tailor treatment to the individual. “This,” he said, “could be another piece of personalized medicine.”
___________________________
1 Berdahl JP et al. Ophthalmology. 2008;115(5):763-768.
2 Ren R et al. Ophthalmology. 2010;117(2):259-266.
3 Fleischman D et al. PLoS One. 2012;7(12):e52664.
4 Wang NL et al. Ophthalmology. 2012;119(10):2065-2073.
5 Varma R et al. Ophthalmology. 2004;111(8):1439-1448.
Meet the Experts
Rand Allingham, MD Richard and Kit Barkhouser Distinguished Chair and Professor of Ophthalmology, Duke University Medical Center, and Senior Consultant, Duke-National University of Singapore, Singapore Eye Research Centre. Relevant financial disclosures: None.
John P. Berdahl, MD In private practice at Vance Thompson Vision in Sioux Falls, S.D., and associate clinical professor of ophthalmology at the University of South Dakota. Relevant financial disclosures: Equinox: C,O; Vittamed: C.
David Fleischman, MD Assistant professor of ophthalmology, University of North Carolina at Chapel Hill. Relevant financial disclosures: None.
Malik Y. Kahook, MD Professor of ophthalmology and the Slater Family Endowed Chair in Ophthalmology at the University of Colorado School of Medicine. Relevant financial disclosures: Equinox: C,O.
For full disclosures and the disclosure key, see below.
|