Download PDF
Thanks to recent studies, ophthalmologists now know more about the molecular, neural, vascular, and systemic processes that contribute to diabetes-related retinal damage and disease progression. Yet the system for classifying and assessing these sight-threatening changes is lagging far behind, a group of researchers wrote in Ophthalmology.1
Their proposed solution: A massive, coordinated, international effort to develop a new staging system that incorporates these advances. “We think that, by updating the severity scales relevant to diabetic eye disease, we will be able to revise the current staging system to help us better predict outcomes in the diabetic eye. And in doing so, we can drive advances that are much needed in both research and clinical care,” said Jennifer K. Sun, MD, MPH, at the Joslin Diabetes Center’s Beetham Eye Institute in Boston.
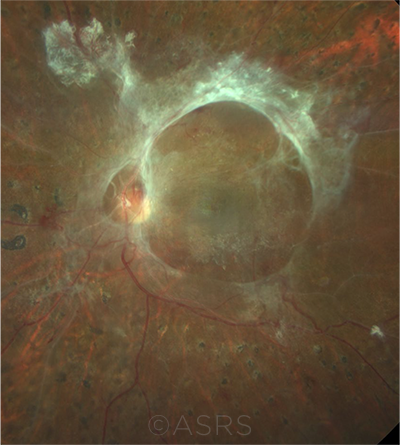 |
LATE STAGE. Ring fibrosis in a patient with proliferative diabetic retinopathy. This image was originally published in the ASRS Retina Image Bank. Rudvij Pandya. Ring Fibrosis Proliferative Diabetic Retinopathy. Retina Image Bank. 2021; Image Number 72551. © The American Society of Retina Specialists.
|
Say hello to DRD. One semantic signal of this new focus comes from the terminology the group has adopted, said coauthor Michael D. Abràmoff, MD, PhD, at the University of Iowa in Iowa City. “We don’t say ‘diabetic retinopathy,’ because that just addresses the vascular component of the retinal damage in diabetic eyes.” Instead, the preferred phrase is now “diabetic retinal disease,” and the narrow acronym DR is being replaced by DRD, he said.
Developing the staging system. Six international panels are currently working on evidence-based recommendations for the metrics to incorporate into an updated staging system for DRD. “Ideally, an updated staging system will address retinal, neural, and vascular pathology and their contributions to visual function in the context of systemic influences such as diabetes type, glycemic control, blood pressure, renal disease, and anemia,” Dr. Sun and her coauthors wrote.1
“As we learn more about diabetes [and its neural components], we have found that neural damage happens early,” Dr. Abràmoff said. While he believes that neural damage occurs earlier than vascular damage does, some of his colleagues think that the neural and vascular damage are “kind of interrelated,” he added. “Everyone in the group has a different view, but we all realize that it’s more than just vascular damage, especially in the macula and the periphery.”
Role for AI. A new system might include disease assessments made with advanced retinal imaging tools, such as ultra-widefield photography, spectral-domain optical coherence tomography (OCT), and OCT angiography, said Dr. Sun, who also serves as chair of diabetes initiatives for the DRCR Retina Network.
Artificial intelligence (AI) tools might be needed to integrate all this complex information into a system that clinical ophthalmologists could easily use, she added. “So much automated decision support is possible with computerized algorithms. That’s one way to potentially make a system that—in and of itself—is quite complex and contains a lot of nuanced information but is easy to use clinically.”
Three years in the making. The roots of the project were laid down in 2018, during an international colloquium cosponsored by the JDRF. That meeting led to the formation of this new initiative to re-envision DRD staging, Dr. Sun said.
—Linda Roach
___________________________
1 Sun JK et al. Ophthalmology. Published online Nov. 17, 2020.
___________________________
Relevant financial disclosures—Dr. Sun: Adaptive Sensory Technology: S; Boston Micromachines: S; Genentech: C; Novartis: C; Novo Nordisk: C,S; Optovue: S; Roche: C,S. Dr. Abràmoff: Alimera: C; Digital Diagnostics: C,O,P; NovaGo: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Abràmoff Alimera: C; Digital Diagnostics: C,O,P; NovaGo: C.
Dr. Finger Liberty Vision: O.
Dr. Foster Aciont: S; Alcon: S,L; Aldeyra: C,S; Allakos: C; Allergan: L; Bausch + Lomb: C,S; Clearside: S; Dompé: S; Eyegate: C,S,O; Genentech: C; Novartis: C,S; pSivida: C,S; Mallinckrodt: S,L.
Dr. Miller Alcon: C; Johnson & Johnson: C,P; Lensar: C; Oculus: C.
Dr. Sun Adaptive Sensory Technology: S; Boston Micromachines: S; Genentech: C; Novartis: C; Novo Nordisk: C,S; Optovue: S; Roche: C,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review