Download PDF
University of Miami researchers have developed an automatic algorithm that combines tomographic images of an in situ corneal graft into a 3-D model that can reveal an otherwise undetectable signal of graft rejection.
Results of their study showed that central thickening of the endothelial/Descemet membrane complex (En/DM) predicted graft rejection at least two months before the clinical diagnosis was made.1 “Transplant surgeons are already using basement membrane to detect rejection in other organs, such as kidney and lung transplants, but they have to do it with a surgical biopsy. We demonstrated we can do it without having to do an invasive procedure, by doing an optical biopsy,” said coauthor Mohamed F. Abou Shousha, MD, PhD.
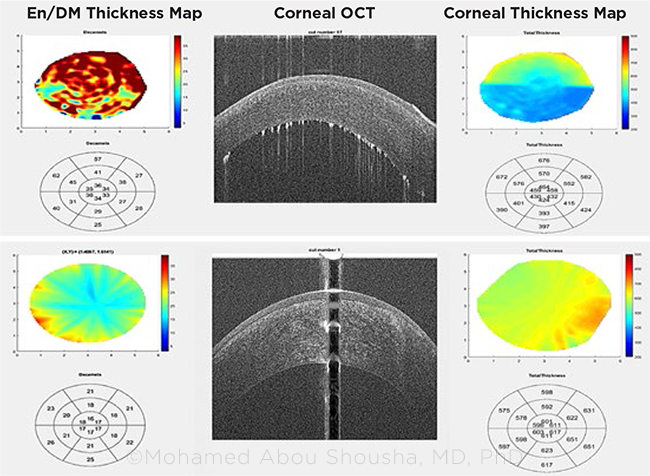 |
OPTICAL BIOPSY. Corneal microlayer tomography of the endothelial/Descemet membrane display shows (top) active corneal graft rejection versus (bottom) a healthy graft. Differences in the thickness maps of the two grafts also are evident.
|
Study findings. For this prospective trial in 60 high-risk cornea transplant patients, the researchers compared the performance of the OCT-based algorithm to the timing of rejection diagnoses made during five postoperative exams (at months 1, 3, 6, 9, and 12).
In eyes that did not develop rejection, analysis of the grafts’ central 2 mm showed that the En/DM thickness was stable through 12 postoperative months. But when the En/DM thickness measured ≥18 μm, the risk ratio for clinical rejection was 6.89 (95% confidence interval [CI]: 2.03-23.4; p = 0.002). Once the En/DMT thickened further to 19 μm or greater, the risk of graft rejection increased by a factor of almost 10.
“The algorithm is detecting the microscopic changes that are happening on the endothelial/Descemet membrane, before rejection becomes clinically apparent,” Dr. Abou Shousha said. “You can see the natural history of rejection rather than a ‘snapshot’ of the one time when the patient visits the physician and receives the diagnosis.”
Mapping the microlayer. The researchers created a system to automatically and reproducibly measure corneal microlayer thickness by doing “optical microlayer tomography” with OCT, Dr. Abou Shousha said.
The group developed software that assembles a series of segmented OCT images, taken radially and centered on the corneal vertex, into color-coded 3-D maps of the layers. The maps are then analyzed, and the En/DM thickness is measured automatically.
What’s next. Further research is needed, in order to demonstrate that the analytic system could be used with all OCT instruments, and FDA approval will be required before commercialization, Dr. Abou Shousha said.
He added that corneal transplant surgeons have told him they are eager to have it available because it could protect patients from going through corneal failure. “We know endothelial cells lost in a rejection episode will never be replaced. So predicting and diagnosing graft rejection early on is extremely important,” Dr. Abou Shousha said. “What we have here is a method for diagnosing subclinical rejection that also would guide treatment decisions.”
—Linda Roach
___________________________
1 Eleiwa T et al. Sci Rep. 2021;11(1):14542.
___________________________
Relevant financial disclosures–Dr. Abou Shousha: NEI: S; Resolve Ophthalmics: O,P.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chen None.
Dr. Jain Foundation Fighting Blindness: S.
Dr. Lois None.
Dr. Sarraf Amgen: C; Bayer: C; Genentech: C,S; Heidelberg: S; Janssen: C; Novartis: L; Optovue: C,S; Regeneron: S; Topcon: S.
Dr. Shousha Heru: O; NEI: S; Resolve Ophthalmics: O,P.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review