By Linda Roach, Contributing Writer, interviewing Rachel R. Caspi, PhD, Emily Y. Chew, MD, Phoebe Lin, MD, PhD, and James T. Rosenbaum, MD
Download PDF
After a decade of studies linking various autoimmune diseases to dysregulation of the gastrointestinal tract’s commensal microbes, scientists are uncovering evidence that gut microbes may underlie an autoimmune eye disease: acute, noninfectious uveitis.
Powerful Influence
Immunology researchers have much yet to learn about the cellular and molecular processes involved and about the lengthy path that activated T lymphocytes take from the gut to the eye. But, based on experiments in animal models of uveitis, they agree that gut microbiota, together with their collective genomes (the “microbiome”), exert powerful influence over immune responses in distant parts of the body, including the eye.
“The microbes that live in our gut educate our immune system. Any diseases in which the immune system is involved are affected by the gut microbiome, for sure. And that includes all noninfectious causes of uveitis,” said James T. Rosenbaum, MD, at Oregon Health & Science University in Portland.
Intestinal dysbiosis. Phoebe Lin, MD, PhD, also at Oregon Health & Science University, agreed. “What we know about noninfectious uveitis is that there’s a disruption in immune homeostasis. Our research has found that in an animal model of autoimmune uveitis there is this intestinal dysbiosis that occurs, and there might even be a certain intestinal microbial signature that is associated with more severe versus less severe disease,” she said.
Drs. Lin and Rosenbaum, along with their Oregon colleague Mark Asquith, PhD, are senior investigators on a team that has been studying the intestinal microbiome’s involvement in autoimmune uveitis since 2011.
 |
|
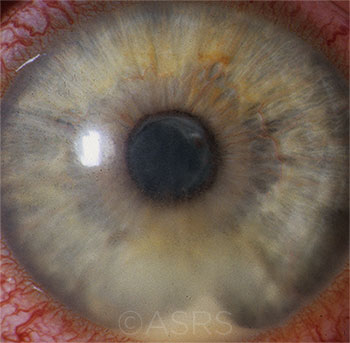
POTENTIAL LINK? HLA-B27 uveitis, shown here, shares a short peptide sequence with certain bacterial molecules. This image was originally published in the ASRS Retina Image Bank. Henry J. Kaplan, MD, and Niloofar Piri, MD. HLA-B27 Associated Uveitis. Retina Image Bank. 2014; Image Number 18312. American Society of Retina Specialists.
|
Adaptive Immunity and Molecular Mimicry
At the National Eye Institute (NEI), the Immunoregulation Section, led by Rachel R. Caspi, PhD, has played a predominant role in developing this emerging picture of autoimmune uveitis. Dr. Caspi and her colleagues published a pivotal research paper in 2015 that linked gut commensals to autoimmune uveitis.1
Activating T cells. Dr. Caspi’s subsequent studies have added support for what now is a plausible hypothesis of how autoimmune uveitis begins. According to this theory, cross-reactive bacterial antigens in the gut—“molecular mimics”—trigger the autoimmune attack on the eye by activating autoreactive T cells that happen to pass through the intestine.
Dr. Caspi’s group performs its uveitis studies in transgenic mice, which develop spontaneous uveitis by age 2 months. The mice have high numbers of peripheral T lymphocytes with a receptor for interphotoreceptor retinoid-binding protein (IRBP).
By itself, this retinal protein is unlikely to be able to activate T cells—in the healthy eye, it is isolated behind the eye’s blood-retinal barrier. But through a series of painstaking experiments, the researchers showed that retina-specific T lymphocytes become activated in the gut, apparently in response to a commensal bacterial antigen that is a molecular mimic of IRBP.
“When lymphocytes are activated, they will not stay in the bloodstream,” Dr. Caspi said. Instead, “they will immediately go to tissue, looking for their antigens.” Thus, she said, “we believe—and I have to emphasize that the experiments we’re doing now are trying to confirm this—that [the lymphocytes] migrate to the eye, break through the blood-retinal barrier, and initiate uveitis. In our current research, we’re trying to address the questions of how do they get out of the gut, where do they go, and how do they end up in the eye.”
Dr. Caspi’s molecular mimicry hypothesis emphasizes adaptive immunity, in which immune cells learn to react against unfamiliar antigens. In addition, her research group is trying to quantify the contribution of innate immunity, in which immune cells are already capable of reacting to antigens on first exposure. “Immune signals are ‘built in’ as components of all bacteria, including gut bacteria,” Dr. Caspi said. “Our preliminary data suggest that they are likely to also be important, potentially acting as an immune adjuvant.”
Understanding the Microbiome
The gastrointestinal mucosa is populated by bacteria, fungi, and viruses, which are estimated cumulatively to greatly outnumber cells in the human body. Research has shown the following:
- Homeostatic balance in the microbiota is maintained through complex microbial-host interactions that activate effector T lymphocytes within the gut (a defense against microbial overgrowth) and regulatory T lymphocytes (to modulate the T-effector cells).
- Dysregulation of this immune machinery can lead to immune-mediated maladies locally (as with inflammatory bowel disease) as well as at distant sites in the body.
- The extraintestinal conditions that have been linked to alterations in the microbiome include a number of diseases, from several forms of arthritis (psoriatic arthritis, rheumatoid arthritis, and ankylosing spondylitis) to atherosclerosis, diabetes, and multiple sclerosis.
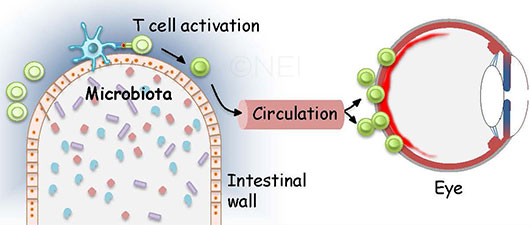
BEYOND THE GUT. As this image illustrates, one hypothesis of autoimmune uveitis suggests that activated T cells pass through the intestine and migrate to the eye, where they break through the blood-retinal barrier.
|
Innate Immunity and Genetics
Other researchers are also investigating the roles that the innate immune response plays when bacteria or bacterial products leave the gut.
Leaky gut? “We are most excited about the possibility that bacterial products escape a leaky gut and distribute widely, promoting inflammation,” Dr. Rosenbaum said. But that cannot be the sole answer, he added, because diabetics and people with rheumatoid arthritis have leaky guts, too, yet they are not susceptible to uveitis.
“So I’m not sure that one size fits all when it comes to autoimmune uveitis,” Dr. Rosenbaum said. “It’s going to have to be something that is somewhat selective, with other factors that contribute. It could be some sort of second hit, like a virus, or genetic factors.”
Genetic factors? Some histocompatibility alleles, including HLA-B27, HLA-A29, and HLA-B51, predispose people to develop various types of acute anterior uveitis, Dr. Rosenbaum said. “HLA-B27 is a major factor in one form. Roughly 40% of acute anterior uveitis is B27-related in the United States,” he said.
Indeed, B27-associated uveitis might illustrate a confluence between Dr. Rosenbaum’s “second hit” idea and Dr. Caspi’s molecular mimicry hypothesis. This is because certain bacterial molecules share a short peptide sequence with HLA-B27, which would enable it to bind to B27 cells.2,3
Thoughts on Treatment
Despite the continuing uncertainty regarding these hypotheses, researchers already have begun looking for potential interventions to prevent the gut from generating uveitic autoimmune cells and sending them to the eye.
Reestablishing balance. Dr. Lin said she favors an immune cell threshold model for uveitis, in which homeostasis requires balance in the gut between regulatory and effector T immune cell types.
“What we think is happening is that, depending on the constituents of the microbiota, the prevalent T cell subsets are altered in the gut and the body. And when you cross a certain threshold, that’s when you’ll develop uveitis, because you don’t have enough regulatory T lymphocytes and [you have] too many pathogenic immune cells. A mimicry event—or events—is also a possibility, as is the leaky gut hypothesis,” she said.
Supporting the microbiome. Early studies in animal models suggest that short courses of oral antibiotics might be used to increase bacterial species that promote regulatory T cells and reduce species that push pathogenic T cell differentiation, Dr. Lin said.
Her lab also found that dietary supplementation with short-chain fatty acids—which normally form in the gut as fermentation metabolites of dietary fiber—dampens down uveitis in 2 ways: 1) The fatty acids increase regulatory T cells in the colon and in cervical lymph nodes, and 2) they reduce migration of effector T lymphocytes to the spleen.4
“Our goal is to take the system back to a more balanced immune system by establishing a bacterial community that is more conducive to more regulatory cells in the immune system,” Dr. Lin said.
Dr. Caspi’s group, in collaboration with scientists at the University of California at Berkeley, is exploring another potential route to modulating effector T lymphocytes via the lipid mediator lipoxin A4. Under healthy conditions, this endogenous bioactive molecule modulates adaptive immune responses to prevent chronic inflammation. When uveitic mice were treated with lipoxin A4, ocular inflammation was reduced.5
Implications for the future. In Clostridium difficile colitis, fecal transplantation is being used to reestablish a healthy microbiome. However, this tactic is unlikely to be a therapeutic option in autoimmune uveitis, Dr. Rosenbaum said.
Nonetheless, “the average clinician needs to know that this [investigation of the microbiome] is the future, and [he or she] needs to be able to respond to questions from patients about diet and probiotics and how this field is evolving,” Dr. Rosenbaum said.
He added, “We’re not at a point right now where we can say if you take a certain probiotic you’re going to be better, if you eat your broccoli and brussels sprouts you’re going to be better, or if you avoid chocolate you’re going to be better. We don’t know that yet. But we will.”
___________________________
1 Horai R et al. Immunity. 2015;43(2):343-353.
2 Scofield RH et al. Lancet. 1995;345(8964):1542-1544.
3 Rosenbaum JT et al. Ocul Immunol Inflamm. 2016;24(4):440-444.
4 Nakamura YK et al. Sci Rep. 2017;7:11745.
5 Wei J et al. Lipoxin A4 dampens T effector cell responses in autoimmune uveitis. Poster C0269 presented at: ARVO Annual Meeting; April 29, 2018; Honolulu.
A Gut-AMD Connection?
Early research suggests that there might be links between gut microbiota and age-related macular degeneration (AMD), which would be mediated by the innate immune system’s responses to bacterial pathogens, Dr. Lin and her colleagues have found.1,2
By profiling the gut microbiomes in stool samples from 85 people with AMD and 49 age-matched control subjects, the researchers found that the microbiota differed between the 2 groups. For instance, those in the AMD group showed an increased abundance of the genus Prevotella and reduced levels of Ruminococcaceae and Rikenellaceae
Another difference was associated with whether the AMD patient was taking the AREDS (Age-Related Eye Disease Study) vitamins, the researchers found. Those who were taking the supplements were found to have more of several intestinal bacteria, most notably the genus Peptoniphilus.
AREDS2 study leader Emily Y. Chew, MD, at the NEI, said scientists there “are interested and may work on some aspects” of the possible gut-AMD connection. However, no plans have been finalized, she said.
___________________________
1 Kiang L et al. Invest Ophthalmol Vis Sci. 2017;58(8):5739.
2 Lin P. Curr Opin Ophthalmol. 2018;29(3):261-266.
|
Dr. Caspi is head of the Immunoregulation Section and chief of the Laboratory of Immunology at the NEI in Bethesda, Md. Financial disclosures: None.
Dr. Chew is director of the Division of Epidemiology and Clinical Applications at the NEI in Bethesda, Md. Financial disclosures: None.
Dr. Lin is assistant professor of ophthalmology at the Casey Eye Institute at Oregon Health & Science University in Portland. Financial disclosures: None.
Dr. Rosenbaum is chief of the Division of Arthritis and Rheumatic Diseases, professor of ophthalmology, medicine, and cell biology, and the Edward E Rosenbaum Professor of Inflammation Research at Oregon Health & Science University. He also is the chair of ophthalmology at the Legacy Devers Eye Institute in Portland, where he holds the Richard Chenoweth Chair. Financial disclosures: AbbVie: C; Eyevensys: C; Gilead: C; NEI: S; Novartis: C,O; OpenBiome: C; Pfizer: S; Regeneron: C,S; Rheumatology Research Foundation: S; Spondylitis Association of America: S; Topivert: C; UCB: C; UpToDate: P.
See the disclosure key at www.aao.org/eyenet/disclosures.