Download PDF
Nearly 34 million people worldwide are currently infected with the human immunodeficiency virus (HIV), and 97 percent of them are in middle- and low-income countries. Ocular complications affect between 50 and 75 percent of these individuals at some point during the course of their illness. These complications include varicella-zoster and herpes simplex virus retinitis, ocular syphilis, ocular tuberculosis, cryptococcal meningitis, ischemic microvasculopathy, and ocular toxic or allergic drug reactions. But, by far, cytomegalovirus (CMV) retinitis is the most significant, causing more bilateral blindness in people with AIDS than any other condition.1
Despite its profound impact, CMV retinitis is now the neglected disease of the AIDS pandemic, according to David Heiden, MD, at Pacific Eye Associates in San Francisco. Clinically, it usually goes undiagnosed and untreated until there is substantial visual loss. Policywise, it is absent from current World Health Organization (WHO) guidelines for the management of HIV in resource-poor areas. And WHO’s “Vision 2020” program doesn’t even mention CMV retinitis.2
“We have to increase awareness of HIV-associated ophthalmic diseases in the developing world,” said Gary N. Holland, MD, at the David Geffen School of Medicine at UCLA. “In resource-limited settings, the focus has been on improved survival with antiretrovirals, which have been provided to HIV-infected individuals by NGOs [nongovernmental organizations] and governments. There has been no focus on eye disease, yet CMV retinitis is a huge problem, especially in Southeast Asia. CMV retinitis is often not diagnosed until serious, irreversible damage has already occurred,” he continued. “This situation is particularly tragic because vision is essential for survival where people have little help with medical care or activities of daily living.”
|
Views Through Two Cameras
|
 |
|
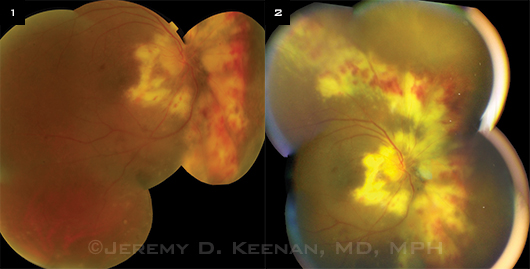
Images of the same retina taken with (1) the Topcon fundus camera, compared with (2) the iPhone Ocular CellScope attachment. Although slightly blurry, the iPhone image may be adequate for diagnosing CMV.
|
Estimated Scope of the Problem
Ironically, although CMV infection is not mentioned in WHO’s policy recommendations, a 2001 review published in the Bulletin of the WHO warned of “an epidemic of blindness,” estimating that between 5 and 25 percent of all HIV-infected patients in the developing world could be expected to develop CMV retinitis.1
With increased availability of highly active antiretroviral therapy (HAART), those numbers are now an overstatement, according to Dr. Heiden, because only patients with advanced HIV infection and low CD4+ counts (less than 50 cells/µL) are vulnerable to CMV retinitis. “But, unfortunately, what we have observed is that about 20 percent of patients in resource-limited settings, even where HIV testing and HAART are readily available, continue to present with advanced HIV infection and vulnerability to CMV retinitis,” he said.
Reported prevalence of CMV retinitis in people living with HIV in resource-limited settings is variable, ranging from less than 5 percent in southern Africa to over 30 percent in Southeast Asia.3 Traditionally, this discrepancy has been attributed to mortality among Africans who die from tuberculosis or other diseases before CMV retinitis can develop. However, recent observations suggest that this hypothesis does not completely explain the discrepancy, and the cause remains unclear.
A recent systematic review found no reduction in the prevalence of CMV retinitis over the past decade.3 “There is a steadily increasing cohort of young patients in middle- and low-income countries made healthy by successful treatment of underlying HIV, yet left permanently blind from undiagnosed or inadequately treated CMV retinitis,” Dr. Heiden said.
Differs From Western Experience
In Western countries, CMV retinitis is now very rare in people living with HIV, though the problem has not disappeared (see the January 2014 feature, "HIV and the Eye: Lessons Learned, Challenges Remain"). In the pre-HAART era, about one-third of patients with AIDS developed CMV retinitis; following the introduction of HAART—as well as widespread testing allowing early detection and treatment of HIV—the incidence of CMV retinitis declined by over 95 percent in the United States.4
The experience in high-income countries has led to a mistaken assumption that CMV retinitis will cease to be a major problem in developing countries as HAART slowly makes its way to them. But the current situation there is not analogous to that of Western countries in the pre-HAART era; it’s actually much worse, according to Dr. Holland: “A lot more patients don’t have access to eye care; there’s even more stigma associated with HIV than there was here [in the United States]; and anti-CMV drugs are unavailable.”
Drugs and IRU: a complex interaction. In the 1980s and 1990s, ophthalmologists in high-income countries didn’t have HAART yet, but they did have anti-CMV drugs. Today, in contrast, resource-poor areas are starting to get HAART but not anti-CMV agents. This exacerbates the problem of immune recovery uveitis (IRU).
If an individual has active, undiagnosed CMV retinitis, the infection continues to proliferate unchecked. If that person is diagnosed with HIV disease or finally treated with HAART—but not anti-CMV drugs—CMV will continue to be present even as the immune system recovers. The patient’s recovered immune system mounts an inflammatory response against the CMV in the eye, resulting in IRU.
“If one can limit the amount of CMV in the eye by diagnosing and treating the retinitis early with anti-CMV drugs, especially before immune recovery occurs, then there won’t be as much virus is the eye. Thus, it is likely that not as much inflammation will be stimulated,” said Dr. Holland.
Barriers to Progress
The fundamentals of successful management of CMV retinitis are screening eye exams in patients with low CD4+ counts and effective anti-CMV treatment with ganciclovir and related compounds (valganciclovir), combined with potent antiretroviral therapy.
Resources lacking for diagnosis, treatment. Because CMV infection does not cause redness or pain, everyone with HIV infection requires routine retinal screening. But limited ophthalmological diagnostic skills and lack of accessible CMV treatment in most settings where HAART is available are major roadblocks. Related issues include health care delivery infrastructure and linkage to care. In addition, treatment is prohibitively expensive and may need to be administered using different approaches from those in the United States (i.e., intraocular ganciclovir injections rather than oral valganciclovir).
Overcoming Diagnostic Limitations
Although aggressive ocular screening is crucial, “the scarcity of ophthalmologists is a primary obstacle,” said Jeremy D. Keenan, MD, MPH, at the Proctor Foundation in San Francisco. “HIV providers rarely know how to examine the retina.”
Training HIV doctors to diagnose CMV retinitis. Reliable diagnosis of CMV retinitis can be made by simply dilating the pupil and looking at the retina with an indirect ophthalmoscope, said Dr. Heiden. “The infected retina has a singular, dramatic appearance, with nicknames like ‘cottage cheese and ketchup’ and ‘pizza pie retinopathy,’” he said. “Diagnosis is quick and simple, and no special tests are needed.”
Can HIV doctors be trained to use an indirect ophthalmoscope to diagnose CMV retinitis, instead of relying on an ophthalmologist? “Learning how to do the retinal exam doesn’t take long, and even if the doctor misses 20 percent of cases, we’re still 80 percent ahead of where we were before,” said Dr. Heiden, who has been working on different methods and curricula for teaching simplified eye skills to AIDS doctors in resource-limited settings throughout the world. There have been 16 four-day workshops to date, trying different methods.
Programs in action. In Myanmar, for example, in collaboration with Doctors Without Borders/Médecins Sans Frontières, Dr. Heiden’s team built a CMV retinitis screening program template. (It remains incomplete, however, because of the lack of ophthalmology back-up needed for patients who have complications such as retinal detachment.) The program works at the primary care level and is national in scope. In addition, said Dr. Heiden, “We’ve trained AIDS doctors who staff the infectious diseases hospitals in the three most affected provinces in China, responsible for 117 million people.”
He has also started work in Eastern Europe, the area in which the AIDS epidemic is increasing most rapidly, where “AIDS patients are unable to gain access to ophthalmologists because of AIDS stigma and because some behaviors (drug addiction and sex work) that can lead to infection are criminalized,” he said.
How telemedicine can help. Dr. Keenan is evaluating telemedicine as another way to improve access to screening. “I think HIV doctors recognize the need for routine screening, but there’s a linkage-to-care problem,” said Dr. Keenan. “In addition to training nonophthalmologists as screeners, it’s important to conduct the screening exams at the point of HIV care.”
In Northern Thailand, Dr. Keenan’s team has compared the diagnostic accuracy of indirect ophthalmoscopy by an ophthalmologist with fundus camera photographs taken by a nonclinician and transmitted to three experts in San Francisco and Los Angeles; the results show that telemedicine is feasible and reasonably accurate. “But even if telemedicine can be conducted by a nonclinician in a busy HIV clinic, the expense of currently available cam-eras—between $8,000 and $30,000 for the Topcon camera used in this research—is still a major limitation for retinal telemedicine in resource-poor settings,” explained Dr. Keenan.
iPhone solution. “Thinking outside the box, Todd Margolis [also at the Proctor Foundation in San Francisco] thought that if an iPhone could be adapted to take retinal photographs of sufficient quality, this might provide a much less expensive, and much more portable, option for telemedicine,” Dr. Keenan said.
His team enlisted the help of a biomedical engineer and medical student to develop an iPhone attachment housing an optical system and external illumination. The engineer also developed an app for taking the photos, which provides autofocus and auto-exposure control. This system provides wider-field images than have been obtained with other, currently available cell phone–based techniques; this is an important feature, as CMV retinitis can develop outside the vascular arcades. Its accuracy is now being tested against that of the Topcon camera Figs. 1, 2 to see whether this lower-cost method is feasible. “Certainly, in some cases, the iPhone has provided good enough quality for the diagnosis of CMV retinitis,” said Dr. Keenan, who hopes to have data to share within a year.
A Diagnostic Bonus of Retinal Screening
Screening for CMV retinitis in HIV-infected patients may provide an additional advantage: The exams may also permit early diagnosis of systemic tuberculosis. “Four hundred thousand AIDS patients die of tuberculosis each year, and in maybe 10 percent of these patients who have advanced AIDS, the diagnosis might be made weeks earlier by simply examining the eyes,” said Dr. Heiden.1
___________________________
1 Bedelu M et al. Clin Infect Dis. 2013;56(2):310-312. |
Making Treatment More Accessible
Reducing cost of medicine. Improving diagnosis is only half of the equation; treatment must also be within reach. The Medicines Patent Pool (MPP) is an international NGO that aims to make HIV treatment more affordable throughout the world by means of agreements with pharmaceutical companies. In August 2013, Roche, the manufacturer of valganciclovir—the easy-to-take oral anti-CMV agent—reached a supply agreement with the MPP to increase access to the drug by making it up to 90 percent cheaper in 138 developing and emerging countries.5 “Unfortunately, the cost is still a barrier, but it’s a start,” said Dr. Heiden.
An intraocular alternative. In the meantime, he relies on the far less expensive ganciclovir intraocular injections and has trained a limited number of HIV doctors to administer these injections. “The injections are not technically demanding to teach or do—easier than starting an IV—but there are fundamental medical reasons why they are inadequate,” said Dr. Heiden. These problems include second-eye involvement and extraocular disease, he added. “Also, for obvious practical reasons, weekly intraocular injection is a much more difficult treatment strategy to reach all those in need of treatment, when compared with pills.”
Keys to Success
Regular screening for CMV retinitis in HIV-positive patients is the most effective way to reduce the blinding complications of the infection. “If we could achieve early diagnosis,” said Dr. Keenan, “we have all the knowledge we need to treat the retinitis effectively. We need to make screening the norm, then increase accessibility to anti-CMV medications.”
“We also need to focus on preventing the inflammatory damage that comes with immune recovery uveitis,” Dr. Holland added. “Getting patients through that period of immune recovery without more eye problems also relies on early diagnosis.”
___________________________
1 Kestelyn PG, Cunningham ET. Bull World Health Organ. 2001;79(3):208-213.
2 Heiden D et al. PLoS Med. 2007;4(12):e334. doi:10.1371/journal.pmed.0040334.
3 McCannel CA et al. Am J Ophthalmol. 1996;121(1):35-46.
4 Ford N et al. Clin Infect Dis. 2013;57(9):1351-1361.
5 Roche and the Medicines Patent Pool Sign Agreement [press release].
___________________________
David Heiden, MD, is a uveitis consultant and general ophthalmologist at Pacific Eye Associates and director of international ophthalmology at California Pacific Medical Center, in San Francisco; and medical director, AIDS Eye Initiative, Seva Foundation, in Berkeley, Calif. Financial disclosure: None.
Gary N. Holland, MD, is Jack H. Skirball Professor of Ocular Inflammatory Diseases at the David Geffen School of Medicine at University of California, Los Angeles. Financial disclosure: Is a member of medical advisory boards for Novartis and XOMA.
Jeremy D. Keenan, MD, MPH, is associate professor in residence at the Francis I. Proctor Foundation of University of California, San Francisco. Financial disclosure: None.