By Linda Roach, Contributing Writer, interviewing David F. Chang, MD, Randy J. Epstein, MD, and Andre J. Witkin, MD
Download PDF
Today, U.S. ophthalmologists who use vancomycin for intracameral antibiotic prophylaxis during cataract surgery must weigh the risk of a rare but potentially blinding complication, hemorrhagic occlusive retinal vasculitis (HORV), against that of endophthalmitis.
Should these surgeons pursue alternatives to vancomycin? In 2013, a large California study confirmed the value, in a U.S. setting, of intracameral cefuroxime to prevent endophthalmitis after cataract surgery1—but access to cefuroxime requires a compounding pharmacy. And ophthalmologists who have been successfully accomplishing intracameral prophylaxis with topical, unpreserved moxifloxacin (Vigamox 0.5%) point out that HORV has not been an issue in their patients.
“There are so many reasons not to use vancomycin,” said Randy J. Epstein, MD, at Rush University Medical Center in Chicago, who has used moxifloxacinintracamerally since 2007. “The CDC is begging people not to use it indiscriminately because of bacterial resistance. And now you have HORV. I think it’s high time to put this issue to bed already.”
The Vancomycin-HORV Link
Last summer, a task force of the American Society of Cataract and Refractive Surgery (ASCRS) and the American Society of Retina Specialists (ASRS) reported on a total of 36 eyes diagnosed with postoperative HORV following uncomplicated cataract surgery and concluded that a rare type III hypersensitivity to vancomycin was the most likely cause.2,3 All but 5 cases occurred since 2013.
The analysis showed that HORV manifests painlessly with a sudden, dramatic decrease in visual acuity, occurring 1 to 26 days (mean, 8 days) after uneventful cataract surgery. The disease is characterized by retinal vascular occlusions, numerous peripheral hemorrhages, and ischemia. The visual outcomes were poor—20/200 or worse in 22 eyes (61%), and no light perception (NLP) in 8 eyes (22%).2 (For more about HORV theories and treatment, see “HORV and Cataract Surgery, Part 1: Update on Theories and Tx.”)
No blanket condemnation. Although members of the ASCRS-ASRS Task Force warned that HORV often causes unilateral or bilateral catastrophic visual loss, they did not recommend that surgeons stop using intracameral vancomycin. “We are hesitant to say, ‘Absolutely stop using it,’ because we think HORV is really rare,” explained Andre J. Witkin, MD, a task force member from the New England Eye Center in Boston. “But it’s unclear whether it’s really worth the risk to use vancomycin in this way.”
Individual decisions. Cataract surgeon and task force cochair David F. Chang, MD, of Los Altos, Calif., noted that approximately half of the cataract surgeons who responded to a 2014 ASCRS member survey were using intracameral antibiotic prophylaxis.4 Among antibiotic users in the United States, vancomycin was the choice of 52%, Dr. Chang said. “Some surgeons believe that vancomycin is more effective against MRSA and other drug-resistant organisms, and they continue to favor this for intraocular antibiotic prophylaxis.”
In his own practice, Dr. Chang said, he became concerned about HORV and its delayed presentation. As a result, he no longer uses vancomycin for intracameral prophylaxis. “I used intracameral vancomycin successfully for 18 years with no cases of bacterial endophthalmitis and no known HORV. However, because I frequently operate on the second eye within 2 weeks of the first, I decided to switch to intracameral moxifloxacin,” he said.
 |
|
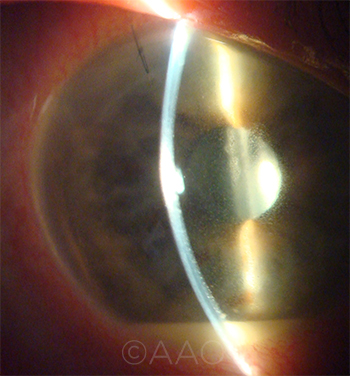
CALCULATING RISK. Cataract surgeons who use vancomycin are now in the difficult position of comparing the potential risk of acute postoperative endophthalmitis (shown here) against that of HORV.
|
What Now?
As the ASCRS survey indicates, half of U.S. surgeons use no intracameral antibiotic prophylaxis.4 But for those who do—and who are now looking to transition away from using vancomycin—here are their current options.
The leading alternative: Vigamox. The second most popular antibiotic for intraocular prophylaxis (31% of those using intracameral antibiotics) in the United States is moxifloxacin, a broad-spectrum, fourth-generation fluoroquinolone.4
In the ASCRS survey, “The majority using intracameral moxifloxacin were injecting unpreserved topical Vigamox by a 7:1 margin over compounded moxifloxacin,” Dr. Chang said.
Dr. Epstein said he regards the decision to repurpose Vigamox in this way as a “no-brainer.” He pointed out, “It’s preservative-free, you know it’s sterile, and there’s no mystery about what’s in the bottle. It’s already compounded to the right concentration for us to use in the operating room. It has a very broad spectrum. And it’s not that expensive.” (See “Overcoming Cost Considerations of Vigamox, below”)
Supporting evidence. When cataract surgeons who lacked easy access to cefuroxime began looking for alternatives a decade ago, moxifloxacin’s easy availability and its rapid, potent bactericidal activity against the most common gram-positive postoperative endophthalmitis pathogens made it an attractive candidate.5 Early clinical studies found that it is well tolerated in the anterior chamber,6-8 and safety issues have not emerged subsequently.
Evidence that moxifloxacin usage reduces the incidence of endophthalmitis includes the following studies.
- Cataract surgeons at Kaiser Permanente in California analyzed outcomes of 315,246 surgeries and found that intracameral doses of cefuroxime and moxifloxacin were equivalent at reducing incidence of postoperative endophthalmitis.9
- In a study conducted by Dr. Chang and Aravind Haripriya, MD, at the Aravind Eye Hospital system in southern India, instituting routine intracameral moxifloxacin prophylaxis in manual small-incision cataract surgeries reduced the incidence of endophthalmitis 4-fold.10
- In a follow-up Aravind study (not yet published), Dr. Chang said that he and Dr. Haripriya compared endophthalmitis rates in 617,453 cataract surgeries, approximately half with and half without intracameral moxifloxacin. “Compared to the 302,815 eyes that didn’t receive intracameral antibiotic, intracameral moxifloxacin reduced the endophthalmitis rate by a factor of 3.5—from 0.07% to 0.02%. This is the strongest clinical evidence to date that intracameral moxifloxacin is effective,” Dr. Chang said.
Caution: No preservatives! It is important to ensure that the moxifloxacin product to be injected is at the proper concentration (1 mg/0.1 mL) and contains no preservatives, in order to avoid toxic anterior segment syndrome (TASS), Dr. Witkin said. “Vigamox is the only one that’s preservative-free, so it’s the only one that you could use,” he said.
What about other fluoroquinolones? It is unknown whether any other fluoroquinolone could be used safely and effectively in the anterior chamber, and Dr. Epstein cautions against trying them. “If you use one of the competing branded fourth-generation fluoroquinolones, they’re not preservative-free,” he said. “With unpreserved moxifloxacin, there’s peer-reviewed literature that documents its safety. Why would you want to put your patients at risk by using something that hasn’t got that kind of a track record?”
What about compounded antibiotics? American ophthalmologists have had access to cefuroxime for intraocular use—but only if they were willing to have it prepared by a compounding pharmacy. Compounded preservative-free moxifloxacin also can be purchased by this route.
Continuing concerns. However, persistent concerns about ensuring sterility, as well as the potential for dilution errors with compounded cefuroxime, has made many surgeons leery of pursuing this option, Dr. Epstein said.
Positive experiences. “I work both in hospital and surgery center settings. One of the hospitals has a system for using compounded moxifloxacin, because it’s cheaper for them than going with Vigamox. And I’ve had no problem with that,” Dr. Epstein said. “In others, I ask the patients to bring in an unopened bottle of Vigamox that I can use for the intracameral injection [see “Overcoming Cost Concerns for Vigamox, below”]. I have found that either system works well for my patients.”
Dr. Chang said he uses moxifloxacin (1 mg/0.1 mL) specifically formulated for intracameral injection by a 503b- certified compounding pharmacy. “I use compounded moxifloxacin from Leiter’s compounding pharmacy, which has a very stable shelf life and is less expensive than a bottle of Vigamox.”
Overcoming Cost Concerns for Vigamox
Dr. Epstein said that the most common reason that other ophthalmologists give for not using intracameral moxifloxacin is economic. “I’m sensitive to the fact that some people are operating in environments where they’ve been told that the cost of providing Vigamox for use during surgeries is an issue.”
Dr. Epstein offered a straightforward solution: “Give the patient a prescription for the Vigamox, and have the patient bring the unopened bottle to the OR on the day of surgery. This way, you get around not only the surgery center’s economic concerns but also all the issues about prescribing and dispensing and [concerns regarding] whether the drug is sterile or not.”
In the OR, the circulating nurse opens that bottle in a sterile manner and squeezes some out from the bottle into a sterile specimen cup. The scrub nurse then aspirates 0.2 cc into a TB syringe, using sterile technique. “At the end of the case, I administer 0.05 cc intracamerally from the sterile syringe, and the patient is given the remainder of the bottle to take home and use topically,” Dr. Epstein said.
|
Stay Tuned
This is by no means the end of the vancomycin dilemma, and surgeons will need to keep abreast of the unfolding HORV story. In the meantime, any cases of HORV should be reported to the ASCRS-ASRS Task Force’s registry.
___________________________
1 Shorstein NH et al. J Cataract Refract Surg. 2013;39(1):8-14.
2 ASCRS-ASRS HORV Task Force. Clinical Alert: HORV Association With Intraocular Vancomycin. Issued July 20, 2016, http://ascrs.org/node/26101. Accessed Jan. 11, 2017.
3 Witkin AJ et al. Ophthalmology. 2015;122(7):1438-1451.
4 Chang DF et al. J Cataract Refract Surg. 2015;41(6):1300-1305.
5 O’Brien TP et al. J Cataract Refract Surg. 2007;33(10):1790-1800.
6 Lane SS et al. J Cataract Refract Surg. 2008;34(9):1451-1459.
7 Arbisser LB. J Cataract Refract Surg. 2008;34(7):1114-1120.
8 Espiritu CR et al. J Cataract Refract Surg. 2007;33(1):63-68.
9 Herrinton LJ et al. Ophthalmology. 2016;123(2):287-294.
10 Haripriya A et al. Ophthalmology. 2016;123(2):302-308.
___________________________
Dr. Chang is clinical professor of ophthalmology at the University of California, San Francisco, and in private practice in Los Altos, Calif. Relevant financial disclosures: None.
Dr. Epstein is professor of ophthalmology and director of the cornea service at Rush University Medical Center in Chicago and CEO of Chicago Cornea Consultants. Relevant financial disclosures: None.
Dr. Witkin is assistant professor of ophthalmology and director of clinical research at the New England Eye Center at Tufts Medical Center in Boston. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.