By Jennifer S. Griffin, MS, Contributing Writer, interviewing Anna K. Junk, MD, Teresa C. Chen, MD, and Nikhil S. Choudhari, DNB
Download PDF
Does your practice use alcohol or hydrogen peroxide to disinfect its Goldmann applanation tonometers? If so, you may risk exposing patients to infection. According to a 2017 Academy Ophthalmic Technology Assessment (OTA), the 3 most commonly used disinfectants are alcohols, hydrogen peroxide, and sodium hypochlorite (bleach); only the last was found to be effective disinfection against adenovirus and herpes simplex virus (HSV), the viruses commonly associated with nosocomial outbreaks in eye care.1
Teresa C. Chen, MD, of Harvard Medical School, who coauthored the OTA, suspects that many physicians are not yet using bleach on prisms. “The common misconception is that alcohol wipes—which are easier—are adequate,” she said.
And good tonometer care does not stop with disinfection. Calibration is important for getting accurate and consistent monitoring of intraocular pressure (IOP), yet calibration protocols are often neglected.2
Learning a few best practices for tonometer maintenance can help ensure safe and effective IOP monitoring in your practice, the experts say.
 |
|
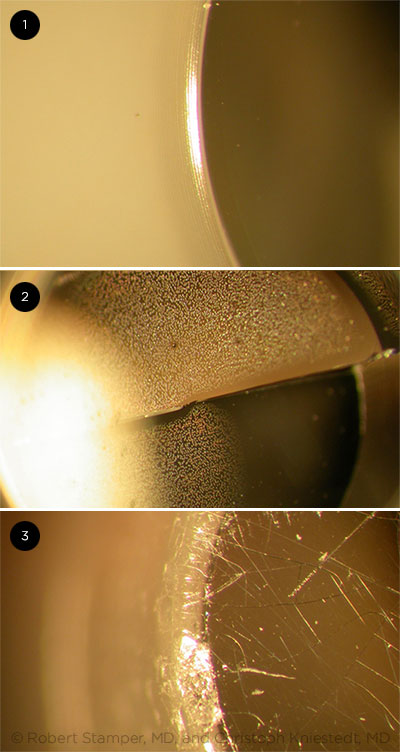
SLIT-LAMP MICROGRAPHS. (1) An undamaged tonometer prism and (2, 3) tonometer prisms that have been damaged over time. Cracks formed in the prism can cause corneal abrasions and may harbor microorganisms or residual disinfectant. Photographs courtesy of Christoph Kniestedt, MD, at the University of Zürich, Zürich, Switzerland, and Robert L. Stamper, MD, Distinguished Professor of Clinical Ophthalmology at the University of California, San Francisco.
|
Disinfecting Reusable Prisms
Because the GAT prism contacts the corneal surface, reusable prisms that are inadequately disinfected between patients can be a source of disease transmission. In fact, tonometer tips have been implicated in clusters of epidemic keratoconjunctivitis.3
The OTA panel evaluated 10 laboratory studies on disinfection of tonometer prisms and concluded that soaking in 10% bleach for 5 minutes afforded the most effective disinfection.1 This finding is consistent with the 2008 Centers for Disease Control and Prevention (CDC) Guideline for Disinfection and Sterilization in Healthcare Facilities4 and with recommendations of tonometer manufacturers.
Microbes of concern. Anna K. Junk, MD, lead author of the OTA and an ophthalmologist at the Bascom Palmer Eye Institute and the Miami Veterans Affairs Healthcare System, stated that most iatrogenic infections in ophthalmic settings can be traced to adenovirus 8, adenovirus 19, or HSV 1. The studies reviewed by the OTA panel address contamination of the tonometer tip with adenovirus 8 and 19, enterovirus 70, HSV-1 and -2, human immunodeficiency virus (HIV) 1, hepatitis C virus (HCV), and prions.
Bleach eliminates a broad spectrum of microorganisms by rapidly oxidizing cell membranes and denaturing proteins, without leaving a toxic residue. The OTA found that a bleach soak is the only method that consistently inactivates adenovirus and HSV—though it may be more troublesome to use than alcohol or hydrogen peroxide.
Although no case of Creutzfeldt-Jakob disease has been linked to GAT, prions are extremely resistant to disinfection, and the incubation period between exposure and observable illness can be several decades. Practitioners should consider using dispos able tip covers or single-use prisms if evaluating a patient with suspected or confirmed prion disease, according to the OTA. However, the risk of prion disease transmission is thought to be extremely low, said Dr. Junk, emphasizing that when you are disinfecting the tonometer prism, “the concerns should be adenovirus and HSV.”
Adenovirus is a causative agent of acute follicular conjunctivitis and epidemic keratoconjunctivitis. And the adenovirus particle is extremely hardy: Desiccated virus can remain viable on surfaces for well over a month, and disinfection with 70% isopropyl alcohol or 3% hydrogen peroxide does not reliably destroy infectivity.1
Safe, aseptic applanation. “The perfect disinfectant would kill viruses commonly involved in infectious outbreaks but would not damage the tonometer tip,” said Dr. Chen. “There isn’t a perfect disinfectant,” she continued, “but diluted bleach inactivates the most commonly involved viruses, and proper disinfection technique can minimize damage to tonometer tips.” The duration of the soak is important. A soak for less than 5 minutes may not inactivate contaminating microbes, and a longer soak is more likely to damage the tonometer tip.
Checking for damage. The OTA panel determined that all disinfectants “have been identified as causing tonometer prism damage and may result in patient injury.”1,5 According to Dr. Junk, over multiple disinfection cycles, the disinfectant may dissolve the glue holding the hollow tip together, causing cracks to form along the rim. These cracks can irritate the cornea and may harbor microorganisms, and the cracked hollow tip can retain disinfectant that could leak out during subsequent applanation. Dr. Junk advised inspecting reusable tips for integrity “under the slit-lamp microscope, every time before use.”
When to discard. Haag-Streit, a manufacturer of tonometer tips, recommends that any reusable tip be discarded 2 years after the first use.6 Dr. Junk noted that all prisms also have “an expiration date after which the tip should be discarded, regardless of the duration of use. Monitoring these dates can be very challenging logistically, especially in a teaching institution.”
Handheld Tonometry Devices
Handheld tonometers include the Tono-Pen XL and Tono-Pen Avia (Reichert) and iCare devices. Cross-contamination and transmission of infectious disease are less of a concern because these tonometers are supplied with single-use tip covers (Ocu-Film, Reichert) or with disposable probe tips (iCare). The iCare does not require calibration.1 And the Tono-Pen Avia requires no regular calibration.2 Dr. Choudhari noted that calibration checks with the Tono-Pen XL are only needed before the first use of the day and when indicated by the instrument display. A common source of inaccurate readings is debris in the handheld tonometer tip.
___________________________
1 http://icare-usa.com.
2 www.reichert.com/eye_care.cfm.
|
Reusable Versus Disposable
As an alternative to reusable prisms, practitioners can applanate with disposable prisms (e.g., Tonosafe, Haag-Streit; Tonomate, Keeler). The choice of tip may be based on personal preference, results of cost-benefit analyses, or concerns about disease transmission. In general, researchers have observed a “gradual but definite shift to the use of disposable prisms worldwide.”7
Dr. Chen said that many physicians continue to utilize reusable tips for 2 reasons: 1) single-use tips can be cost-prohibitive [Tonosafe and Tonomate are more than $1/prism], and 2) “some physicians are uncertain whether the disposable tips have the same accuracy as the reusable tips.”
Cost comparison. In a cost-benefit analysis of reusable versus disposable prisms conducted nationally in the United Kingdom, Jasani et al.7 noted substantial savings (£2 million annually) with reusable GAT prisms. (However, the authors did not account for the added costs and time associated with disinfecting tonometer tips.) Tsai et al.8 found that Tonosafe prisms were approximately 8-fold more costly than reusable prisms.
At Veterans’ Affairs Medical Centers nationally, Dr. Junk explained that implementation of CDC guidelines4 involved either transitioning to disposable tips or submitting used reusable tips to a central sterilization unit. “We were looking at purchasing roughly 1,000 reusable tips to make sure we would not run out given a 2-day turnaround for sterilization. We chose disposable tips.” She also noted that disposable tips are a mainstay among glaucoma faculty at Bascom Palmer.
Accuracy differences? In a study of 326 patients at general and specialty eye care clinics, IOP measurements of Tonosafe disposable prisms and GAT with reusable prisms were found to correlate closely, and repeated measurement variability was similar for the 2 modalities.9 In a prospective study of 100 patients with glaucoma, Tsai et al.8 demonstrated good correlation in IOP results between Tonosafe and reusable GAT prisms.
Safety differences? Sterile, single-use tips have the obvious safety benefit of minimizing disease transmission between patients due to insufficient disinfection. Nonetheless, caution should be exercised even when applanating with a disposable prism. In an assessment of Tonosafe prism use at the Sussex Eye Hospital (United Kingdom), 16 of 35 questionnaire respondents admitted to touching the applanating face of the disposable prism prior to use, and Staphylococcus epidermidis and S. aureus were cultured from briefly touched prisms.10 This occurred despite respondents indicating that the reusable prisms were easier to handle and were unlikely to be touched on the applanating face during preparation.10
Calibrating the Tonometer
In GAT, calibration error (CE) typically is assessed at 0, 20, and 60 mm Hg, but widely accepted guidelines on CE check frequency are lacking, said Nikhil S. Choudhari, DNB, at the VST Glaucoma Centre of L.V. Prasad Eye Institute in Hyderabad, India.
Acceptable level of error. Dr. Choudhari and colleagues assessed 132 GATs at their institution and found only 4% compliance with manufacturer-recommended CE tolerance for GAT at 20 mm Hg, even though their bioengineering department performs annual servicing and recalibration on the devices.11 However, Dr. Choudhari noted that manufacturer limits are stringent in a clinical sense and may actually represent an industrial (ISO) convention. The levels of acceptable CE set by the World Glaucoma Association (WGA) and the Asia Pacific Glaucoma Society (APGS) are more likely to be achievable in clinical practice. The WGA recommendation is within ± 1 mm Hg at all testing levels, and the APGS guideline is within ± 2 mm Hg at 0 mm Hg, ± 3 mm Hg at 20 mm Hg, and ± 4 mm Hg at 60 mm Hg.11
“I am of the opinion that the acceptable level of CE should depend on the severity of glaucoma,” said Dr. Choudhari. “A wider range of CE might be acceptable in early to moderate glaucoma, but error in the measurement of IOP should be minimal in advanced disease.” His practice generally applies a CE tolerance limit of ± 2 mm Hg.
Calibration frequency. In a study of 100 ophthalmology residents, 85 acknowledged that they never check the GAT for CE, and only 7 stated that they perform CE checks at the start of each clinical session.2 Dr. Choudhari emphasized the importance of checking the GAT frequently for CE. He and others have found that as many as “20% of tonometers between 1 and 10 years of age and as many as 50% of tonometers more than 10 years of age can develop CE in a month.”12 Dr. Choudhari recommended that new tonometers be checked biannually in the first year. Tonometers more than 1 year old should be checked monthly.12
Dr. Choudhari and others have proposed a “screening approach” in which CE is determined frequently, but only at 0 mm Hg.13 This is the testing level at which CE check weight bars are not involved. The screen can be performed quickly and easily on a weekly or even daily basis and had a good negative likelihood ratio of 0.11 when the less stringent APGS guideline was applied.13 Dr. Choudhari asserted that this simplified approach “should not be used as a substitute for the monthly checks of CE with the weight bars, especially in the context of advanced glaucoma.”
Sources of error. GAT calibration can be affected by dust accumulation or fluctuations in humidity or temperature. Dr. Choudhari also noted, “The GAT is a balancing instrument, and any tilt in the surface on which the tonometer is mounted can cause errors in measurement of IOP.” In addition, operator-related factors can increase the likelihood of CE, Dr. Choudhari explained. “For example, an inadvertent backward push on the tonometer tip during cleaning may cause wear to the spring mechanism.”
Correcting the problem. Dr. Choudhari said that there are 2 ways to address CE in GAT. “If an instrument is found to have an unacceptable CE, the ophthalmologist should send the instrument to the manufacturer for repair.” Alternatively, he and others have described a technique by which a bioengineer can address CE in-house.
Best Practices
Dr. Junk gave the takeaway message for disinfection of the reusable prism after every patient: “Wipe the tonometer tip clean. Soak in 10% bleach for 5 minutes. Rinse with water, and let dry. Check for cracks every time before use with 16 × magnification on slit lamp.”
Based on the current evidence, doing so—coupled with frequent calibration of the GAT—can improve patient safety and precision of IOP measurements at the slit lamp.
___________________________
1 Junk AK et al. Ophthalmology. 2017;124(12):1867-1875.
2 Kumar N, Jivan S. Eye (Lond). 2007;21(6):733-734.
3 Massey J et al. MMWR Morb Mortal Wkly Rep. 2016;65:382-383.
4 Rutala WA et al. Centers for Disease Control and Prevention. 2008. www.cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf.
5 Kniestedt C et al. Acta Ophthalmol Scand. 2005;83(1):129-130.
6 Haag-Streit. Guide to Goldmann Reusable Prisms. 2013. www.haag-streit.com/fileadmin/Haag-Streit_USA/Diagnostics/tonosafe/download/Prism-Checking-USA.pdf.
7 Jasani KM et al. BMJ Open Ophth. 2017;1:e000019.
8 Tsai AS et al. J Glaucoma. 2014;23(8):521-525.
9 Thomas V et al. Eye (Lond). 2011;25(5):651-656.
10 Rajak SN et al. Eye (Lond). 2006;20(3):358-361.
11 Choudhari NS et al. Ophthalmology. 2009;116(1):3-8.
12 Choudhari NS et al. J Glaucoma. 2016;25(11):908-913.
13 Choudhari NS et al. J Glaucoma. 2016;25(10):812-814.
___________________________
Dr. Chen is associate professor of ophthalmology, Harvard Medical School, and Massachusetts Eye and Ear Infirmary, Glaucoma Service, both in Boston. Financial disclosures: None.
Dr. Choudhari is an ophthalmologist at VST Glaucoma Centre, Dr. Kallam Anji Reddy Campus, L.V. Prasad Eye Institute, Hyderabad, Telangana, India. Financial disclosures: None.
Dr. Junk is associate professor of clinical ophthalmology at Bascom Palmer Eye Institute and Miami Veterans Affairs Medical Center, both in Miami. Financial disclosures: None.