By Annie Stuart, Contributing Writer, interviewing Shan Lin, MD, Kyoko Ohno-Matsui, MD, PhD, and Sarwat Salim, MD, FACS
Download PDF
By 2050, about half the world’s population will have myopia.1 “In some parts of the world, these rates are already approaching 90%,” said Sarwat Salim, MD, FACS, at the New England Eye Center in Boston. Compounding these troubling statistics are population-based studies that show a 2- to 4-fold increased prevalence of glaucoma in myopic eyes, she said, with an even stronger association for those with moderate-to-high myopia.
This lends urgency to the task of identifying myopic patients who are most at risk for developing glaucoma, but there isn’t a simple solution for doing so. The structural and functional defects in myopic eyes are difficult to distinguish from those caused by glaucoma, said Dr. Salim. “What’s most important is to focus on various risk factors for glaucoma and get baseline ancillary tests, such as OCTs, visual fields, and disc photos; to be aware of clinical features of both myopic and glaucomatous optic nerves; and to follow these patients longitudinally.”
 |
|
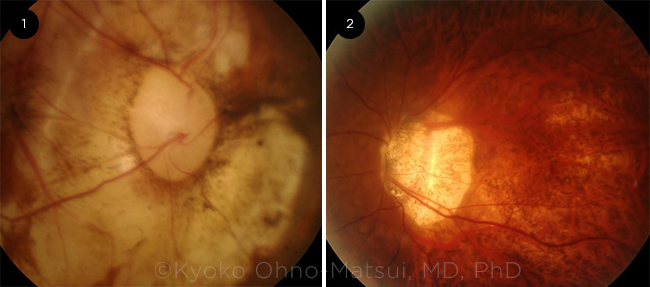
DISC ABNORMALITIES. Potential optic disc changes seen in myopic patients include: (1) an acquired megalodisc and (2) an extremely tilted disc.
|
Why Does Myopia Increase Glaucoma Risk?
“We don’t have a good understanding of the connection between myopia and glaucoma, and this is an area of active research,” said Dr. Salim. It is believed that the myopic eye’s structural abnormalities, mainly those related to laminar collagen fibers, increase the risk of developing glaucoma, she said.
For instance, axial length can be greater than 25.5 mm in moderate-to-high myopia, versus 23 mm in normal eyes, she said, and this elongation causes stretching and thinning of the lamina cribrosa and thinning and weakening of the sclera. These structural changes, combined with morphological changes of the optic nerve, make these patients’ optic nerves more susceptible to damage related to intraocular pressure (IOP) elevation.
IOP and myopia. The Singapore Epidemiology of Eye Diseases study found that IOP and myopia may act synergistically on the development of primary open-angle glaucoma (POAG).2 The authors looked at varying levels of IOP and severity of myopia—comparing one group without myopia to another with moderate-to-high myopia. “Those with moderate-to-high myopia and IOP of 20 mm Hg or greater were four times more likely to develop POAG,” said Dr. Salim.
IOP and axial length. The Singapore researchers also looked at the correlation between IOP and axial length. Eyes with high IOP and axial length greater than 25.5 mm were 16 times more likely to develop POAG when compared with those with shorter axial length and lower IOP. “That’s clinically important information. As we care for patients, we should pay attention not only to refractive status and the level of myopia, but also to axial length, which can be easily measured in clinics and can provide invaluable information for future glaucoma monitoring and therapeutic decisions,” Dr. Salim said.
Ethnicity. Because of a higher rate of myopia in Asians, the detection of glaucoma is especially important in these populations, said Kyoko Ohno-Matsui, MD, PhD, at Tokyo Medical and Dental University’s Advanced Clinical Center for Myopia. Researchers haven’t yet confirmed, however, that race is a risk factor for glaucoma in high myopes, said Shan Lin, MD, at the Glaucoma Center of San Francisco.
Additional concerns. “A significant risk factor for developing glaucoma, including in highly myopic patients, is a family history of glaucoma,” he said. “In individuals with moderate-to-high myopia, glaucoma can develop earlier than in regular POAG—often in young or middle adulthood. And the risk appears relatively high, even with borderline IOP, around 20 or 21 mm Hg.”
A less well-known risk for myopic glaucoma is Flammer syndrome, a vascular dysregulation more common in women and Asians, said Dr. Lin. In addition to myopia, symptoms of this syndrome may include cold hands and feet, systemic hypotension, low body mass index, decreased thirst, difficulty falling asleep, migraines, tendonitis, and increased sensitivity to pain, odors, and certain drugs.3 “Migraines,” he pointed out, “are a well-known risk factor for normal tension glaucoma.”
Structural and Functional Assessments
Interpreting glaucomatous changes in highly myopic eyes is particularly difficult, especially in those with pathologic myopia, where there’s myopic maculopathy and posterior staphyloma, said Dr. Ohno-Matsui.
Multiple challenges. In high myopes, “fundus features can be difficult to observe because the optic disc is severely tilted and deformed,” she said. “And a coexisting large conus and lesions of myopic maculopathy can make it more challenging to use the Humphrey field analyzer.” Generalized retinal thinning can also complicate quantitative assessment of the nerve fiber layer defect (NFLD) or ganglion cell complex (GCC) on OCT, she said.
“Swept-source OCT centered on the optic disc can detect optic disc pits and conus pits that may relate to the development of glaucomatous visual field defects in patients with pathologic myopia,” she said. “However, we basically rely on Goldman perimetry for detecting visual field defects in eyes with pathologic myopia.”
Myopic optic nerves. It’s critical to know the characteristics of myopic and glaucomatous changes to the optic nerve—and to remember that there can be an overlap between the two, said Dr. Salim. She noted that compared with glaucomatous optic nerves, myopic optic nerves tend to be larger, with a disc area greater than 3.25 mm2. In addition, they are vertically elongated with an oval shape, which can cause a tilt in the optic nerves that obscures both the temporal and nasal rims. They also have very shallow diffuse cupping, an increased incidence and extent of beta zone peripapillary atrophy, and a decreased retinal nerve fiber layer (RNFL) thickness.
OCT strategies. “We need to interpret OCT findings very carefully,” said Dr. Salim. “To begin with, high myopes have decreased average RNFL at baseline, which may also have an atypical distribution. RNFL bundles can shift temporally, which can cause thinning in nasal sectors and thickening in temporal quadrants, complicating assessment for glaucoma.”
It’s also important to pay attention to the size of the optic nerves and peripapillary atrophy on OCT printouts, she said. “In myopic eyes with significant peripapillary atrophy, the circle centered around the optic nerve for RNFL measurements can overlap the atrophy, leading to erroneous measurements” and inaccurate interpretation.
Normative databases. Overall, OCT has not been particularly reliable for detection of glaucoma in people with moderate-to-high myopia, because these patients are not well represented in the normative reference database, said Dr. Salim. The optic disc and nerve tissue is so abnormal in these patients, said Dr. Lin, that this database may cause you to misclassify them—either by categorizing them as patients who do not have glaucoma or, conversely, by attributing their damage to glaucoma when it is more related to myopia.
But researchers are now developing normative databases for people with myopia, Dr. Lin said. He also noted that a Korean group found that using a myopic-specific database helped to discern areas of defect in eyes with myopic glaucoma when applying OCT color probability codes.4 The Nidek OCT machine has a myopic-specific data-base for detecting NFLD and GCC for low-to-moderate myopia, added Dr. Ohno-Matsui. “But using this database to assess the thickness of RNFLor GCC may not be useful for highly and pathologically myopic eyes.”
Optic disc stereophotography. In general, optic disc photos are no longer routinely done, said Dr. Salim. “But for this particular patient population, I find them extremely valuable, especially since OCT may not be reliable. These photos provide a more objective assessment and are useful for longitudinal follow-up to see if any new and subtle structural changes [attributable to glaucoma] are taking place.”
Visual field defects in myopia. Although visual fields play a major role in evaluating eyes at risk for glaucoma, clinicians must be aware that many visual field defects can occur with myopia alone. These may include a large blind spot, nasal step, arcuate defect, or paracentral defect, said Dr. Salim. “These visual defects in myopia occur in patterns that are similar to those in glaucoma, but they may be unrelated to it.”
In one study, nearly 80% of myopic patients without glaucoma had visual field defects. More than 15% had nasal step and about 28% had paracentral defects.5 “Arcuate scotomas, which may correspond to thin or nonexistent rim tissues at the inferior and superior poles of myopic optic nerves, occurred in 35.5%,” Dr. Salim said.
Another study followed 16 Chinese men who had either glaucoma or were glaucoma suspects, 14 of whom had had baseline visual fields.6 “After seven years of follow-up, none had progression of optic nerve or visual field defects, irrespective of their IOP reduction,” said Dr. Salim. The takeaway message? The defects might have been due to myopia, not glaucoma, she said, cautioning that, at times, myopic eyes may be at risk of being overtreated for glaucoma.
Visual field strategies. In highly myopic eyes, macular lesions impair central vision, which causes poor fixation during a functional evaluation, said Dr. Ohno-Matsui. Microperimetry may overcome this problem, as clinicians can assess the retinal sensitivity at the point they would like to measure, she said. “Goldmann perimetry taken by a skilled technician is the best strategy for detecting characteristic visual field defects in myopic glaucoma. I highly recommend that patients with high or pathologic myopia have Goldmann perimetry at least once a year to avoid underdiagnosis of glaucoma. However, in highly myopic eyes without large conus or myopic macular atrophy, the Humphrey field analyzer may be an option, too.”
Dr. Lin advises getting visual fields more than once a year—as often as every three to six months. “It’s imperative that we follow these patients closely because progression of glaucoma can sometimes be rapid.” He has had myopic patients in their 20s, 30s, and 40s with severe visual field defects who had a confirmed diagnosis of glaucoma. However, he emphasized that it’s important not to dismiss the possibility that defects can be attributed to high myopia alone.
To help differentiate between the two, Dr. Lin recommended alternating between 24-2 or 30-2 visual fields and 10-2 visual fields. The latter provides a more closely spaced mapping of the central 10 degrees, thus helping clinicians spot any small defects in central vision at an earlier stage.
 |
|
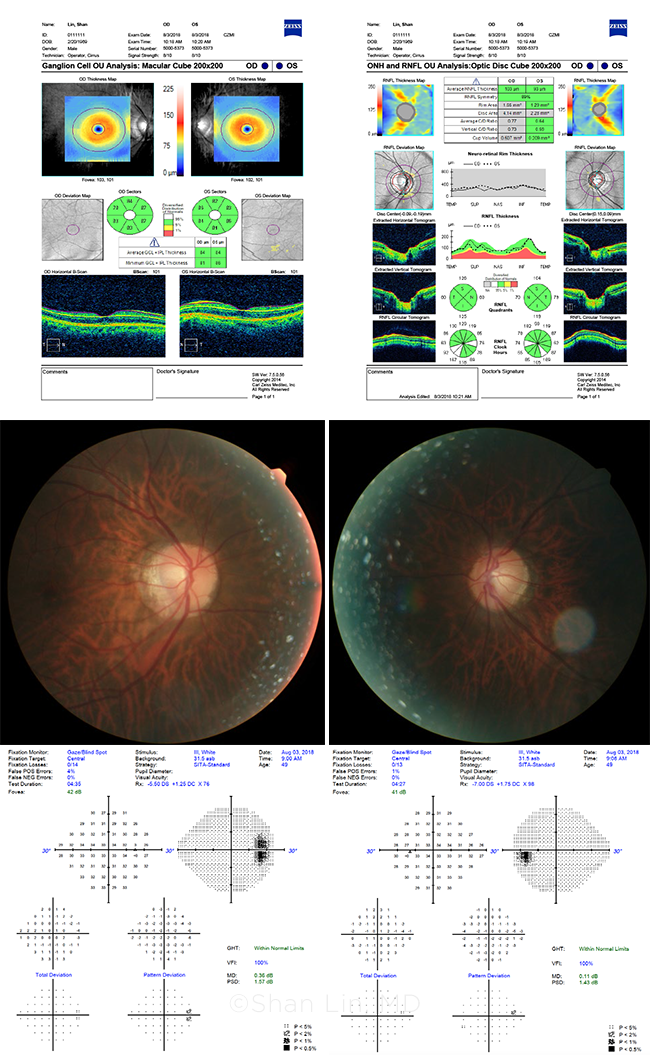
MYOPIA PLUS GLAUCOMA? These images are from a glaucoma suspect whose nerves look suspicious, but whose tests are “normal.” The patient? Dr. Lin himself.
|
__________________________
1 Holden BA et al. Ophthalmology. 2016;123(5):1036-1042.
2 Tham YC et al. Sci Rep. 2016;6:19320.
3 Konieczka K, Erb C. EPMA J. 2017;8(4):327-332.
4 Seol BR et al. Am J Ophthalmol. 2017;183:147-155.
5 Kumar RS et al. J Glaucoma. 2012;21(5):281-286.
6 Doshi A et al. Ophthalmology. 2007;114(3):472-479.
__________________________
Dr. Lin is co–research director at the Glaucoma Center of San Francisco. Relevant financial disclosures: None.
Dr. Ohno-Matsui is professor and chairperson of ophthalmology and visual science at Tokyo Medical and Dental University (TMDU) in Tokyo, Japan. She is also chief of the Advanced Clinical Center for Myopia at TMDU. Relevant financial disclosures: None.
Dr. Salim is professor of ophthalmology, vice chair of clinical and academic affairs, and director of the glaucoma service at the New England Eye Center, Tufts University School of Medicine, in Boston. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Lin Aerie: C,L; Bausch + Lomb: C,L; Eyenovia: C; Iridex: C.
Dr. Ohno-Matsui Bayer Healthcare: C; CooperVision: C; Santen: C.
Dr. Salim Aerie: C,L.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|