Download PDF
In a pair of studies, researchers at the Bascom Palmer Eye Institute in Miami have confirmed that persistent hypertransmission defects (hyperTDs)—defined as those with a greatest linear dimension of 250 μm or more—can serve as an independent risk factor for the progression of drusen to geographic atrophy (GA) in dry age-related macular degeneration (AMD).1,2
On OCT, hyperTDs appear as bright regions due to increased light transmission into the choroid where the retinal pigment epithelium (RPE) is attenuated or absent. Previous research has found that hyperTDs could be seen during the progression from intermediate AMD to late-stage AMD.
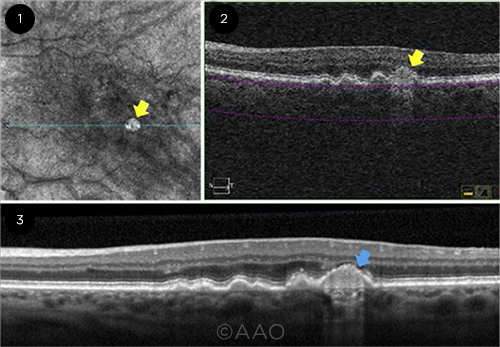 |
EVIDENCE. (1) A hyperTD shows as a bright lesion on en face OCT (yellow arrow). (2) Corresponding OCT B-scan shows the increased choroidal hypertransmission (yellow arrow). (3) Nascent GA (blue arrow), identified on OCT B-scan.
|
En face SD-OCT. Using en face spectral-domain OCT, the researchers evaluated 81 patients (157 eyes). Thirty-nine hyperTDs were documented in 22 patients (27 eyes) and classified as either persistent (26 lesions) or transient (13 lesions) over a three-year period. The presence of the lesions was confirmed on corresponding OCT B-scans.1
The hyperTDs (≥250 μm) were associated with nascent GA, the analysis found. Because of the high negative predictive value (≥94%), the researchers concluded that these persistent hyperTDs could be used as a stand-alone precursor for the progression from intermediate AMD to GA.
En face SS-OCT. In a larger study, the researchers found that a persistent hyperTD significantly increases the risk of developing GA in eyes with intermediate AMD.2
For this study, the researchers used en face swept-source OCT to assess 134 patients (190 eyes). All told, 73 eyes had at least one hyperTD at baseline or follow-up. However, those with hyperTDs >250 μm (n = 31) were more likely to progress to GA than were those with smaller lesions (n = 42). Overall, the researchers estimated, the risk of developing GA increased by 80-fold once a hyperTD >250 μm appeared.
Next steps. The Bascom Palmer researchers are planning a treatment trial to slow disease progression in eyes with intermediate AMD, using the appearance of persistent hyperTDs as the clinical trial endpoint, said Philip J. Rosenfeld, MD, PhD, coauthor of both studies. They’ve also developed and trained a machine learning algorithm that can detect hyperTDs on en face images.
High praise for en face OCT. Dr. Rosenfeld credits the Classification of Atrophy Meeting (CAM) group, an international team of AMD and retinal imaging experts, for emphasizing the importance of OCT over autofluorescence imaging for diagnosing GA.
“I love the simplicity of the method,” Dr. Rosenfeld said. “En face OCT imaging is the most accurate and efficient way to diagnose and monitor disease progression in dry AMD when compared with other imaging modalities. There’s no need to use color fundus imaging, autofluorescence imaging, or infrared reflectance imaging to detect the earliest formation and subsequent growth of GA. We can do it all with a single OCT raster scan.”
—Miriam Karmel
___________________________
1 Shi et al. Ophthalmol Retina. 2021;5(12):1214-1225.
2 Laiginhas R et al. Am J Ophthalmol. Published online Nov. 13, 2021.
___________________________
Relevant financial disclosures: Dr. Rosenfeld—Apellis: C,O; Bayer: C; Carl Zeiss: C,S; BoehringerIngelheim: C; Chengdu Kanghong Biotech: C; Gyroscope Therapeutics: S; OcuDyne: C,O; Ocunexus (inflammX): C; Regeneron: C; Stealth BioTherapeutics: S; Unity Biotechnology: C; Valitor: O; Verana Health: O.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Jeffery None.
Dr. Liebmann Allergan: C; Diopsys: O; Galimedix Therapeutics: C,O; Genentech: C; Infocus Capital Partners: C; NEI: S; Qura: C,O; Sustained Nano Systems: C,O,P; Thea Pharmaceuticals: C.
Dr. Rosenfeld Apellis: C,O; Bayer: C; Carl Zeiss: C,S; Boehringer Ingelheim: C; Chengdu Kanghong Biotech: C; Gyroscope Therapeutics: S; OcuDyne: C,O; Ocunexus (inflammX): C; Regeneron: C; Stealth BioTherapeutics: S; Unity Biotechnology: C; Valitor: O; Verana Health: O.
Dr. Wirta AbbVie: S; Aerie: C,S; Allergan: C,S; Allysta: S; Bausch + Lomb: S; Eyenovia: C,S; Jennivision: S; Nicox: S; Novaliq: S; Novartis: C,S; Ocuphire: S; Ora: S; Orasis: S; Osmotica: S; Oyster Point Pharma: C,S; Qlaris: S; Santen: C,S; TearCare: S; Tersus: S; Visus: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review