By Rebecca Taylor, Contributing Writer, interviewing Amitha Domalpally, MD, PhD, Srinivas Sadda, MD, and David Sarraf, MD
Download PDF
Despite new software iterations, optical coherence tomography angiography (OCTA) remains challenged by artifacts that can disrupt volumetric data and the clarity and usefulness of images.1
Rapid advances in the quantitative data outputs from OCTA technology have spurred its use in clinical trials as well as hopes of wider use. But OCTA “is still a relatively new technology, still rapidly evolving,” cautioned SriniVas Sadda, MD, at the Doheny Eye Institute in Los Angeles. “There are new types of artifacts specific to OCTA, and these are not necessarily going to disappear completely, regardless of software advances.”
A noisy problem. Dr. Sadda noted that each device uses its own hardware platform and software algorithms. “You can’t just interchange the data between devices, because they use different approaches to extracting and processing information,” he said. “Even in the perfect situation, with no errors during the acquisition or processing of data, there is going to be some ‘noise’—and if you repeat the same scan a minute later, it’s not going to be exactly the same.”
Thus, Dr. Sadda said, ophthalmologists need to be able to decipher whether a change they see in the images is meaningful or not.
 |
|
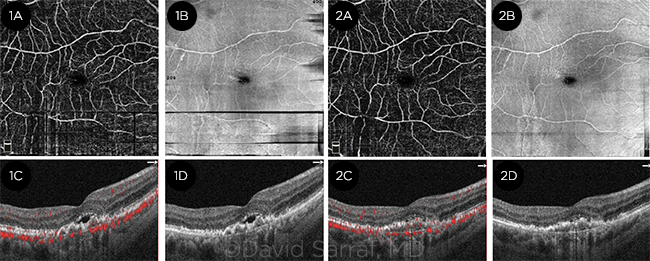
COMPARISON. Horizontal and vertical bands of motion artifacts are noted in the first set of scans with en face OCTA and en face OCT. These are much improved in the second set, thanks to reduced eye movement.
|
Elucidating the Issue
A study published earlier this year in JAMA Ophthalmology detailed the prevalence of various artifacts in 406 OCTA images of eyes with diabetic retinopathy.2 Researchers at the University of Wisconsin-Madison documented at least one artifact in 395 images (97.3%); artifacts severe enough to disrupt the reliability of quantitative outputs were found in 217 images (53.5%). Given the prevalence of artifacts and lack of research into the link between artifacts and quantitative outputs, the authors cautioned against basing clinical decisions on OCTA at this time.
“There was very little being reported in the literature about artifacts, because people need to sit with the images and look at them for a long time to understand the artifacts,” said study coauthor Amitha Domalpally, MD, PhD. “Severe artifacts can affect the data, and we found them in more than 50% of the images. We asked, can we look at these images and their vascular density measures and say—reliably and confidently—that the nonperfusion is truly there; or is it because of the artifact? We felt that severe artifacts inhibited us from reliably extracting those measurements.”
The findings spurred additional research: Dr. Domalpally is now evaluating the specific impact of artifacts on measurements. The researchers are taking scans, inducing artifacts, then removing them “so that we can see the image with and without the artifacts to compare the measurements,” she said. “We are looking to identify what can be measured in the OCTA images to help the research go forward.”
Assessing the Problem
In the University of Wisconsin study, independent graders analyzed the images for various artifacts and ranked them on a 0-to-3 severity scale.1
In addition to the outcomes noted below, it should be noted that only 11 of the 406 images in the study contained no artifacts. In addition, the two OCTA systems used provided high sensitivity (97% to 99%) but relatively low specificity (37% to 46%). Finally, tilt and refraction shift artifacts had not been previously associated with quantitative output changes in earlier studies of OCTA.
Prevalence of Artifacts
Eye movement 93.1%
Defocus 74.9%
Shadow 62.3%
Tilt 50.5%
Z-offset 43.8%
Refraction shift 31.8%
Segmentation error 24.6%
Decentration 21.4%
Projection 6.7%
___________________________
1 Holmen IC et al. JAMA Ophthalmol. 2020;138(2):119-126.
|
Common Artifacts With OCTA
Motion artifacts. OCTA devices take multiple scans from one location, comparing them from one moment to the next. With stable fixation, what has changed is assumed to be blood flow. But movement of a patient’s eye, head, or body can cause blood flow decorrelations or fluctuations in the scan, known as motion artifacts.
“Of the various OCTA artifacts that can degrade an image, the most prominent are related to motion,” said David Sarraf, MD, at the Stein Eye Institute in Los Angeles. “Any loss of fixation due to poor vision or because the patient is not comfortably sitting at the machine can result in artifacts that can degrade the image.”
With each algorithm iteration, technicians have been able to produce better quality images, Dr. Sarraf said. “Some of the new algorithms have tracking systems designed to limit motion artifacts. But if there are vertical or horizontal white lines, silhouetting, or crisscrossing lines on the image, the technician should repeat the scan; the acquisition time for scans is relatively short.”
Segmentation artifacts. OCTA is based on 3-D data viewed on 2-D screens. Analyzing a particular plane (more precisely, a thin slab) depends on where that slab begins and ends.
“Most devices automatically decide where a border should be based on where different layers of vessels should be positioned,” said Dr. Sadda. “Different machines may differ in where they divide the retina into different layers.”
With traditional OCT, ophthalmologists are used to viewing images in a cross-section (e.g., a B-scan). “You can look at your OCTA data the same way, by looking at the flow information superimposed on the B-scan,” said Dr. Sadda. “But when we’re doing quantification, we are generally using en face OCTA images from different slabs, and that’s where segmentation artifacts can manifest.”
Equally concerning, Dr. Sadda said, is the role of disease in disrupting these automated algorithms. “If a disease takes out a layer of the retina, where should the boundary be?”
Projection artifacts. Projection artifacts arise when blood flow of superficial layers of the retina is projected onto deeper structures below. If there’s motion in a superficial retinal vessel, for example, there can also be motion in a shadow behind it.
“OCTA doesn’t distinguish whether it’s the original structure or a shadow; it’s just reporting a change at one location from scan to scan,” said Dr. Sadda. “The trickiest part of looking for a projection artifact is that you won’t see it clearly everywhere below that structure; the projection artifact will be most apparent wherever you have the next bright object below.”
Even with a device’s projection artifact tool enabled, Dr. Sadda advises cautious interpretation and healthy skepticism when something looks like blood flow where it’s unexpected. “Look at your structural OCT en face image, and if there’s a brightly reflective structure there, then that heightens your suspicion,” he said. “Then look at the B-scan with the flow overlay. If you see flow in those deeper layers that perfectly matches flow above it, then be pretty suspicious that’s projection artifact. If there’s no flow above it, and you just see the flow in that deeper structure, then it’s less likely to be a projection artifact.”
All three experts suggest that it takes time to correctly interpret OCTA images.“You have to have the tools and the review station set up in your office so you can view your OCTA data in this way, and it requires a few minutes to look for these things,” Dr. Sadda said.
Signal attenuation artifacts. For OCTA to work, light has to penetrate multiple layers to scan the deeper structures of the retina. “If there’s a loss of signal by the time it reaches the deeper layers such as the choriocapillaris, it can result in a signal attenuation artifact,” said Dr. Sadda.
“Loss of signal impacts your ability to detect whether or not there is flow, which is why your technician has to maximize the signal strength,” he said. “If the signal quality changes a lot between visits or acquisitions, that can artificially impact the appearance of the vessels and their measurements.”
And such changes can lead to faulty diagnosis. The image “can suddenly look like much worse flow or vessel density,” Dr. Sadda said, “but maybe that patient developed a significant cataract over the years that made the signal much worse.”
How to Improve OCTA Interpretation
Use of “four-up” image review. Dr. Sadda recommends looking at four images displayed together as the ideal method for spotting artifacts such as segmentation and projection.
“When you look at your OCTA data, don’t just look at the en face slab; pay attention to the corresponding B-scan and the structural OCT,” he said. “Essentially you’re looking at a ‘four-up’ image display: your OCTA en face, your structural OCT en face from that same location, your standard OCT B-scan, and the B-scan with the OCTA data as the flow overlay.”
Technician training. Adequate training can help reduce artifacts such as projection and motion, for example, by having the patient move their eye to clear vitreous obstructions or repeating scans when obvious artifacts or loss of signal are apparent.2
“Train your technicians to look for potential problems such as evidence of motion artifacts, and if they see discontinuities, have them repeat the scan,” said Dr. Sadda. “Train them to maximize the signal by using artificial tears or having patients blink, and if a scan doesn’t meet minimal signal requirements for reliable data, repeat the scan.”
Patient instruction. Coaching patients may also help. “The technician needs to coach patients not to move their eyes and make sure their heads are comfortable within the chin rest and headband,” said Dr. Sarraf.
Patient selection. There is one caveat to be aware of, Dr. Sarraf said: “For patients with severe central vision loss and severe macular pathology, such as advanced disciform scar or late-stage macular degeneration due to geographic atrophy, fixation can be very difficult.” As a result, he said, “these patients are not optimal for OCTA testing.”
A Role for AI?
As with many technologies, artificial intelligence (AI) is making inroads into OCTA devices. The experts offered two possible outcomes of this combination:
Production of better images. The algorithms that allow OCTA to differentiate blood flow changes at both superficial and deep levels might eventually be used to alert a technician to the need for a repeat scan. “Maybe we’ll reach a stage in which there are algorithms that can be inserted into the camera, [prompting it to] take a picture and to tell the technician right away to retake the image,” said Dr. Domalpally.
Prediction of disease progression. In another scenario, AI might be used to boost OCTA’s ability to predict the progression of disease.
“OCTA is an amazing diagnostic modality that helps us detect choroidal neovascularization and choroidal ischemia in various degenerative and inflammatory disorders, and it’s an important resource to identify nonperfusion and ischemia, especially in retinal vascular diseases,” said Dr. Sarraf. However, he pointed out, “the predictive power of OCTA has fallen short. We haven’t been able to develop any reliable way to use OCTA to predict progression and activity of disease. We’re starting to look into AI, which can integrate information on a much grander scale, as a potential way to use OCTA to predict outcomes.”
___________________________
1 Spaide RF et al. Prog Retin Eye Res. 2018;64:1-55.
2 Holmen IC et al. JAMA Ophthalmology. 2020;138(2):119-126.
___________________________
Dr. Domalpally is research director at the Fundus Photograph Reading Center, Department of Ophthalmology & Visual Sciences, University of Wisconsin-Madison. Relevant financial disclosures: None.
Dr. Sadda is president and chief scientific officer of the Doheny Eye Institute and professor of ophthalmology at the David Geffen School of Medicine, University of California, Los Angeles. Relevant financial disclosures: Carl Zeiss Meditec: S; CenterVue: C,S; Heidelberg: C,S; Nidek: S,L; Optos: C,S; Topcon: S,L.
Dr. Sarraf is clinical professor of ophthalmology in the Retinal Disorders and Ophthalmic Genetics Division at the Stein Eye Institute, University of California, Los Angeles. Relevant financial disclosures: Heidelberg: S; Optovue: C,L; Topcon: S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Domalpally NEI: S; Research to Prevent Blindness: S.
Dr. Sadda 4DMT: C; Amgen: C; Allergan: C; Carl Zeiss: S; Centervue: C,S; Heidelberg: C,S; Nidek: S,L; Novartis: C; Merck: C; Regeneron: C; Roche/Genentech: C; Optos: C,S; Topcon: S,L.
Dr. Sarraf Amgen: C; Bayer: C; Genentech: C; Heidelberg: S; Novartis: L; Optovue: C,L; Regeneron: S; Topcon: S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|