Download PDF
Should more cataract surgeons set aside their doubts and add intracameral antibiotic injections to their endophthalmitis-prevention routines? U.S. holdouts on the issue lost one of their chief objections to this mode of prophylaxis at the beginning of 2013, when California researchers confirmed the results of a 2007 landmark European study.
The U.S. study found that an intracameral injection of cefuroxime at the end of surgery was effective at preventing endophthalmitis,1 as it was in the European trial.2 When the California team analyzed more than 16,000 cases of cataract surgery, they found that the rate of endophthalmitis dropped 22-fold when intracameral antibiotics were used.
“The take-home message is that there is now clear evidence that intracameral antibiotics are a benefit,” said study coauthor Neal H. Shorstein, MD, with Kaiser Permanente.
|
Making the Case
|
 |
|
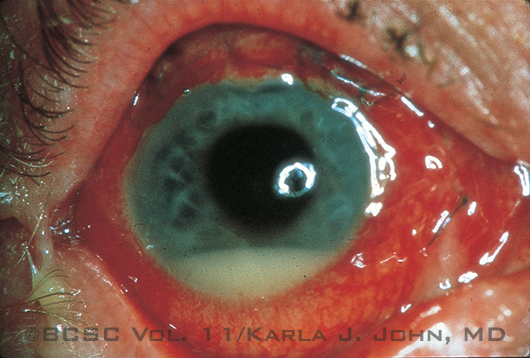
Recent evidence indicates that the rate of endophthalmitis (shown here) drops substantially when intracameral antibiotics are used.
|
Increasing Acceptance?
Many American cataract surgeons have resisted the European study’s conclusions as inapplicable because of procedural differences between Europe and the United States. Instead, they have opted for topical prophylaxis, despite weaker evidence of efficacy.3 But the latest data may bring about a change, observers say.
David F. Chang, MD, at the University of California, San Francisco, said he has noticed new attention being paid to the practice of injecting intracameral antibiotics to prevent endophthalmitis. “As more clinical studies are published, interest among U.S. surgeons is definitely increasing.”
Surgeons who remain skeptical about this method of preventing infection might want to reevaluate their stance, said Randy J. Epstein, MD, at Rush University.
“I think the European and California studies have provided us with ample evidence of the safety and efficacy of this route of antibiotic administration,” said Dr. Epstein.
Safety Issues
Compounding concerns. The problem with using cefuroxime? In a word: compounding. “There is no commercially available, FDA-approved drug right now. And that’s really the huge hurdle,” Dr. Shorstein said. “I think at this point the skeptics are more concerned about the compounding issue than about the efficacy.”
“Of all of the endophthalmitis prophylaxis measures in use, preoperative Betadine [povidone-iodine] solution and direct intracameral antibiotic injection are supported by the strongest evidence,” Dr. Chang said. “However, in order to begin injecting cefuroxime into the anterior chamber, the surgeon first must find a way to formulate it properly and safely, such as using a well-run compounding pharmacy.”
Since his study was published, Dr. Shorstein said, he has been kept busy responding to e-mails and phone calls from ophthalmologists. Many of the calls he has fielded have come from surgery centers, where staff members “want to know how we compound the antibiotics, because it wasn’t in the original article.”
As it happens, Dr. Shorstein and his colleagues have an advantage that many cataract surgeons do not: an in-house compounding pharmacy that they trust to perform the painstaking process of preparing the medication for ocular use.
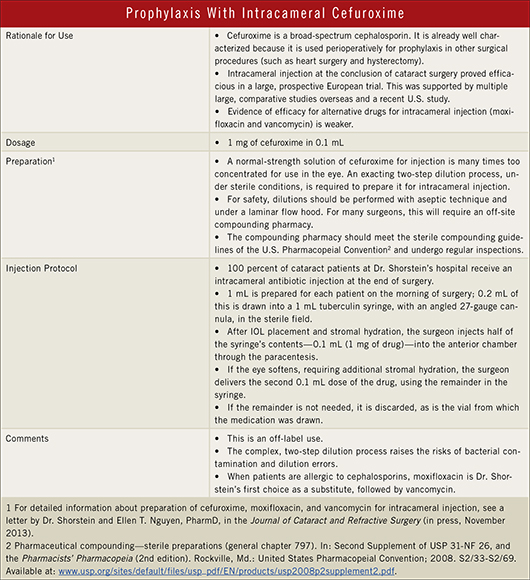
Strict protocol for use. Cefuroxime, which is a broad-spectrum cephalosporin, is approved and labeled in the United States for intravenous and intramuscular use. It is sold to pharmacies in bulk powder form and, when reconstituted as recommended, produces a solution of 750 mg of drug per 7.5 to 8 mL.
In contrast, the standard intracameral injection volume is 0.1 mL, containing 1 mg of cefuroxime. Moreover, a portion of the high-concentration solution must be withdrawn and diluted a second time with nonpreserved normal saline, then transferred 1 mL at a time into single-use vials for the operating room. All steps require strict aseptic technique and a laminar flow hood, Dr. Shorstein said.
Single-dose solution. In Europe, concerns about the risks of dilution error and microbial contamination during this lengthy process were eased last year, when a French company began selling single-dose cefuroxime, called Aprokam, for intracameral injection. (The drug is also sold under the names Prokam and Aprok.)
Dr. Chang and others have called publicly for a U.S. drug company to follow suit; so far, however, none has taken this step.
Prevent Toxic Errors
Antibiotic solutions prepared for intracameral injection should be free of preservatives or other additives, Dr. Shorstein warned.
An outbreak of toxic anterior segment syndrome (TASS) was reported after a compounding pharmacist—who was diluting topical moxifloxacin for intracameral use—used Moxeza, which contains a preservative and other additives, instead of Vigamox, which is preservative free.1
Proper dilution of cefuroxime also is essential (1 mg/0.1 mL), to avoid an intracameral injection containing as much as 100 times the recommended dose. TASS and permanent visual loss have been reported after dosing errors.2-4
___________________________
1 Mamalis N. “TASS associated with intracameral antibiotic injection,” May 8, 2013. www.ascrs.org/Press-Releases; accessed Sept. 27, 2013.
2 Olavi P. Acta Ophthalmologica. 2012;90(2):e153-154.
3 Qureshi F, Clark D. J Cataract Refract Surg. 2011;37(6):1168-1169.
4 Delyfer MN et al. J Cataract Refract Surg. 2011;37(2):271-278. |
Alternatives to Cefuroxime
Vancomycin: Effective against MRSA. “A frequently overlooked human pharmacokinetics study from Liverpool4 demonstrated surprisingly high and prolonged aqueous levels of vancomycin following intracameral injection at the conclusion of cataract surgery,” Dr. Chang said. “I have used intracameral vancomycin for more than 12 years. At the conclusion of surgery, I inject 0.1 mL [1 mg] of diluted vancomycin that is mixed by the nursing staff in the OR on the morning of surgery.”
What about fears that using a last-ditch antibiotic in this way might increase bacterial resistance in exposed ocular bacteria? “Because such a tiny dose of vancomycin is injected into a sterile and isolated ocular compartment, I believe that the theoretical risk of inducing drug resistance should be tiny,” Dr. Chang said. “The Liverpool study provides good evidence that effectively high aqueous levels are sustained well beyond 24 hours with this dosage, and vancomycin does cover” methicillin-resistant Staphylococcus aureus (MRSA).
(click to expand)
Moxifloxacin: Logical and easy. Topical moxifloxacin (Vigamox 0.5 percent), which is widely used in the United States to treat infections of the ocular surface, has characteristics that make it attractive to surgeons who are leery of compounded cefuroxime but convinced that injecting an antibiotic into the chamber is beneficial, Dr. Epstein said.
“Since we have a [formulation of] moxifloxacin that is nonpreserved, this is the path of least resistance,” he said. (See “Prevent Toxic Errors.”)
At least one study has reported that moxifloxacin, a broad-spectrum, fourth-generation fluoroquinolone, is well tolerated in the anterior segment.5 Moreover, surgeons can trust that an unopened bottle of Vigamox will be both sterile and properly formulated, Dr. Epstein said.
The process of sourcing and handling the medication in the OR is not difficult, he added. “I order that the patient be given preoperative Vigamox on the table, just prior to surgery. That way they have their own, brand-new, sterile bottle that’s been ordered for them. The scrub nurse withdraws some in a sterile manner prior to eye-drop administration.”
If the center where he will be operating balks at paying for the drug, Dr. Epstein writes a prescription for it preoperatively and instructs the patient to bring the unopened bottle to the OR on the day of surgery. “The nurses open that bottle in a sterile manner, and the scrub nurse withdraws 0.2 cc from the bottle into a syringe, using sterile technique. The eyedrops are administered preoperatively. Then, at the end of the case, I administer 0.05 cc intracamerally from the sterile syringe.” The patient is given the rest of the bottle to take home.
More than 4,000 eyes after he began this routine, Dr. Epstein has this verdict: So far, so good. “I’ve never had a patient who has received intracameral moxifloxacin develop endophthalmitis thus far.”
For a video on injection protocol, see this month's home page.
___________________________
1 Shorstein NH et al. J Cataract Refract Surg. 2013;39(1):8-14.
2 Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons. J Cataract Refract Surg. 2007;33(6):978-988.
3 Gower EW et al. Cochrane Database Syst Rev. 2013 July 15;7:CD00634.
4 Murphy CC et al. Br J Ophthalmol. 2007;91:1350-1353.
5 Lane SS et al. J Cataract Refract Surg. 2008;34(9):1451-1459.
___________________________
David F. Chang, MD, practices in Los Altos, Calif., and is a clinical professor of ophthalmology at the University of California, San Francisco. He reports no related financial interests.
Randy J. Epstein, MD, is professor of ophthalmology at Rush University Medical Center in Chicago and CEO of Chicago Cornea Consultants. He is a consultant for Alcon.
Neal H. Shorstein, MD, practices in Walnut Creek, Calif., with Kaiser Permanente. He also serves as the associate chief of quality for Kaiser Permanente’s Northern California Diablo Service Area. He reports no related financial interests.