Download PDF
A biodegradable sustained-release drug implant for glaucoma treatment proved as effective as topical drops at the 6-month mark of a 2-year clinical trial.1 Bimatoprost SR was well tolerated and provided rapid, sustained reduction of intraocular pressure (IOP) in patients with mild to moderate visual field loss.
The implant was developed to address poor adherence, which is endemic in glaucoma. “Applying drops is not only challenging for many patients, leading to poor compliance, but requires higher doses to get through the cornea,” said Richard A. Lewis, MD, at Sacramento Eye Consultants.
“The implant is fundamentally the same as the Ozurdex [dexamethasone] implant used for posterior segment disease,” Dr. Lewis said. In both cases, the active drug is slowly eluted over time through the Novadur (Allergan) biodegradable polymer platform.
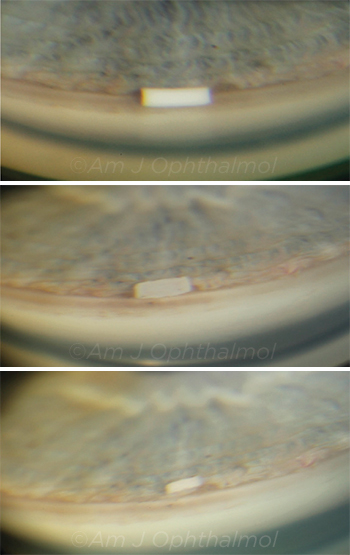 |
IMPLANT IN PLACE. Gonioscopic photographs of a 10 μg bimatoprost sustained-release implant in the anterior chamber of the eye of a patient with open-angle glaucoma at (top) 2 weeks, (center) 9 months, and (bottom) 12 months after injection.
|
The study. In this phase 1/2 study, 75 open-angle glaucoma patients received varying doses of Bimatoprost SR intracamerally in the study eye. The fellow eye received topical bimatoprost 0.03% once daily. Among the findings:
- IOP reduction was observed in implant eyes as early as day 1 and at all subsequent visits through month 6.
- Through week 16, mean IOP reduction from baseline ranged from 7.2 to 9.5 mm Hg depending on dosing, compared with 8.4 mm Hg in topically treated eyes.
Safety. More than half (52.0%) the study eyes experienced adverse events (typically conjunctival hyperemia), compared with 30.7% of fellow eyes. But study eye events mostly occurred within 2 days of the injection procedure and were transient.
Later-onset conjunctival hyperemia occurred more often in topically treated eyes (17.3%) compared with implanted eyes (6.7%).
Patient-reported outcomes. The high level of patient satisfaction surprised Dr. Lewis. At week 12, nearly 80% said they would likely have another implant procedure. The implant lasts 4 to 6 months.
“This is the beginning of a new era in drug delivery for many diseases,” Dr. Lewis said. “Implants optimized for specific diseases will allow better treatment as well as better compliance.”
—Miriam Karmel
___________________________
1 Lewis RA et al. Am J Ophthalmol. 2017;175:137-147.
___________________________
Relevant financial disclosures—Dr. Lewis: Aerie: E (part time); Alcon: C; Allergan: C; AVS: C; Glaukos: C; Ivantis: C. This study was sponsored by Allergan plc, maker of Novadur.
For full disclosures and disclosure key, see below.
More from this month’s News in Review