Download PDF
Intraoperative aberrometry promises to fine-tune cataract surgery results through aphakic refraction—but is it making a difference in practice?
In a sea of new high-tech tools for ophthalmic surgery, intraoperative wavefront aberrometry is an innovation that some believe could enable cataract surgeons to send nearly all of their patients home with less than 0.5 D of pseudophakic refractive error without breaking the bank.
Intraoperative aberrometry is intended to reduce residual refractive error through aphakic refraction, which allows the surgeon to confirm or revise the IOL power choice reached via preoperative biometry, optimize the lens location, and tailor arcuate corneal incisions to the eyes astigmatic needs.
"This is going to be the next horizon in ophthalmic surgery,” said Steven I. Rosenfeld, MD, a cornea, refractive, and cataract surgeon in Delray Beach, Fla.
Initially, intraoperative aberrometry was used in eyes that had undergone refractive surgery, which makes conventional biometry methods less predictable, said Sonia H. Yoo, MD, at the Bascom Palmer Eye Institute. But refractive cataract surgery practices around the country that have adopted this technique have found that it has increased the number of people with previously unoperated eyes who choose a presbyopia-correcting or toric intraocular lens (IOL). This trend may hint at what lies ahead.
"I could imagine a time when every single patient who undergoes lens surgery has intraoperative aberrometry and refraction performed as a standard of care,” Dr. Yoo said. “You would take the picture and, basically, get the IOL power without having to put in a fudge factor or otherwise guesstimating."
1 Device Approved, Another Awaits
The only intraoperative aberrometer currently available in the United States is the Optiwave Refractive Analysis (ORA) system (WaveTec), and a second-generation device is on the horizon.
Meanwhile, Clarity Medical Systems hopes to win FDA marketing approval by the end of 2013 for its Holos intraoperative aberrometry system for cataract surgery. Both ORA and Holos are designed to be mounted on the operating microscope and function in effect as an autorefractor.
Like the ORA, Holos gathers optical wavefront and refraction data to verify the preplanned IOL power and help the surgeon choose the size and location of incisions to correct astigmatism. According to David F. Chang, MD, a Los Altos, Calif., ophthalmologist who worked with the Holos in its early days, the device uses a proprietary wave front-analysis method that is faster than the interferometry used by ORA, allowing it to measure and compute the wavefront refraction more rapidly.
"Holos is like viewing a video, while ORA is more like viewing a snapshot,” Dr. Chang said. "You could literally dial a toric IOL into alignment according to an instantaneous display of the residual cylinder axis and amount. You could immediately assess the effect of your phaco incision, of widening or deepening an LRI, of lifting the lid speculum, or of over- or underinflating the globe."
Until Holos arrives, however, ORA occupies a unique niche as the only commercially available device that can directly measure the eye’s aphakic and pseudophakic refractive status while the patient remains on the operating table.
The Backstory of Aberrometry
ORA is the latest descendant of a handheld device that Tsontcho “Sean” Ianchulev, MD, MPH, invented during his ophthalmology residency at the Doheny Eye Institute.1 The goal was to develop a biometry tool that did not require axial length or keratometry measurements to produce a reliable refraction, he said. Dr. Ianchulev, who holds the original patents on intraoperative refractive biometry, is now an associate clinical professor at the University of California, San Francisco, and a member of WaveTec’s scientific board.
ORA’s most recent predecessor was the ORange wavefront aberrometry system, which ORA replaced two years ago.
Mounted on the operating microscope, ORA uses infrared light and Talbot-Moiré interferometry (which analyzes moiré patterns produced by light passing through two gratings), optimized for the aphakic state (–5 to +20 D), to do a “whole-eye” assessment of the optical system’s refractive power. It takes 40 measurements in less than a minute. The system displays the scans in sequence, then combines and analyzes data from the central 4 mm to determine optimal IOL power for the eye.
Why Intraoperative Measures
Intraoperative autorefraction addresses some of the uncertainties of cataract surgery planning, said Dr. Rosenfeld.
Reduces guesswork. “Up until now you’ve had to take your best guess, based on preoperative biometry, as to what’s the best strength lens to implant, or your best guess, based on marking the cornea before surgery, as to what’s the best axis for a toric lens. Now, you can actually keep rotating the IOL until the aberrometer shows that you’ve minimized the astigmatism. Or you can exchange the lens if the power is not accurate,” he said.
Dr. Ianchulev added, “In a very noninvasive way and a very simple way, one can deliver superior [refractive] outcomes without changing the surgical routine. Instead of having an average error of more than 0.75 D, and in some cases surprises of 1 and 2 D, you can reduce that tremendously.”
Detects posterior astigmatism. Aphakic refraction takes into account factors like posterior corneal astigmatism, Dr. Ianchulev said. If not detected by conventional biometry, posterior astigmatism can cause unanticipated refractive error after cataract surgery, Koch and colleagues reported last year.2 In their study of 715 eyes in 435 patients, the researchers found that posterior corneal astigmatism averaged –0.30 D. Consequently, they concluded, picking a toric IOL power based solely on the anterior surface measurements:
- Would underestimate total astigmatism by 0.22 at the 180-degree meridian (and by more than 0.50 D in 5 percent of the eyes)
- Could cause toric overcorrection in eyes that have with-the-rule astigmatism
- Could cause undercorrection in eyes with against-the-rule astigmatism
Helps with picky patients. Predictability of refractive outcomes is especially important to patients who are paying extra for presbyopic or toric IOLs. In addition, post-LASIK and post-PRK patients have high expectations for visual outcomes, as they’ve already proved by having chosen to undergo refractive surgery. Their reshaped corneas often stymie conventional biometry, yet they also are among the unhappiest patients if there is residual error, refractive surgeons say.
Fewer enhancements needed. In a survey by Shareef Mahdavi, whose firm, SM2 Strategic, in Pleasanton, Calif., consults for the medical device industry, ophthalmologists who regularly use the device said that their enhancement rates fell by nearly half, from an overall average of 10 percent to 5.3 percent.3 This anecdotal evidence remains to be corroborated in clinical trials.
Other Imaging Approaches
In addition to wavefront aberrometry, other types of imaging and guidance systems are in use—or being developed for—cataract and corneal surgery.
Anterior Segment OCT in the OR?
Intraoperative imaging with high-speed optical coherence tomography (OCT) is most often employed by retina surgeons, but its use in the anterior segment also is being studied.
“At Bascom Palmer we’re using intraoperative OCT with corneal surgeries like DALK, DSEK, and DMEK, and we’re looking at it to help us with developing corneal curvature maps, corneal thickness maps, and ultimately, with IOL power,” said Dr. Yoo. “In the future it also could potentially be used for determining effective lens position.”
A large, NEI-funded study of OCT integrated in the operating microscope is currently recruiting (NCT01588041). According to the study description, “The primary outcome of this project is to integrate optical coherence tomography with the surgical environment through novel advances in OCT technology; automated tracking of surgical instruments and tools; and fusion of OCT controls, images and measurements into a seamless interface for the surgeon.” Scheduled to conclude in 2018, the study aims to include more than 200 anterior segment cases in addition to 500 retina cases.
Surgical Guidance Systems
“Companies are moving toward acquiring technology that they can integrate into their microscopes to make the whole process of intraoperative imaging more seamless,” said Dr. Yoo. The desired outcome is analogous to “GPS navigation in the eye.”
TrueVision 3D Surgical. The TrueVision 3D Visualization and Guidance System generates individualized guidance overlays and surgical templates based on patient data gathered at the slit lamp. The system includes a real-time eye tracker to assure that the overlay and the eye remain aligned during surgery.
Callisto eye (Carl Zeiss Meditec). This system was cleared in the United States in spring 2013. It incorporates biometric information from the IOLMaster to generate template overlays for IOL placement, toric alignment, capsulorrhexis, and incision location on the tabletop monitor screen and in the eyepieces of the OPMI Lumera 700 surgical microscope. However, preop diagnostic data still must be transferred to the OR manually via USB.
SMI Ophthalmic/Alcon. The German company SensoMotoric Instruments (SMI) makes video-based eye-tracking systems that can operate at speeds of up to 1,250 Hz, with a processing latency of less than 0.5 ms. SMI’s “surgery pilot” technology (added to the LuxOR operating microscope) will create a way to link cataract patients’ eye coordinates automatically from presurgery diagnostics to intrasurgery treatment, the company said. This technology was acquired by Alcon late in 2012.
|
Device Drawbacks
However, the device has a few drawbacks and requires extra surgical maneuvers.
For some, a long learning curve. In Mr. Mahdavi’s survey, 20 percent of 101 respondents said it took them more than 100 cases to feel comfortable with ORA. However, 38 percent said they made this transition in the first month, after completing fewer than 30 cases.3
Readings difficult in some eyes. Dr. Yoo noted that ORA has difficulty capturing data from some highly aberrated corneas, such as eyes that have previously undergone penetrating keratoplasty or radial keratotomy with small optical zones.
Added time. When asked about how much time intraoperative aberrometry added to their surgical time, surgeons reported a range from 15-30 seconds to 5-6 minutes.3 Until a recent hardware upgrade, each intraoperative wavefront scan with ORA added around one minute to the case time. Consequently, multiple scans during a single case—for instance, to check the refractive effect of tiny nudges in a toric implant’s axial location—wreaked havoc with patient flow, users complained. “One minute can feel very long,” said Dr. Rosenfeld. “Nonetheless, I’d rather wait a minute and have that information than not have it.”
At the 2013 American Society of Cataract and Refractive Surgeons (ASCRS) meeting, WaveTec announced a hardware upgrade (VerifEye), which is intended to speed up the process and add continuous video and refractive readouts.
Consolidate, please. Dr. Yoo said she is looking forward to a time when the field of refractive cataract surgery is no longer littered with an assortment of high-tech tools that don’t communicate with one another. “I think in the future these technologies as they continue to develop will merge and become more integrated with the microscope, and more integrated into devices like the IOLMaster and Lenstar,” she said. “Sometimes the next breakthrough in technology is just a matter of bringing together existing technologies so that they are easy to use.”
(click to expand)
Surgical Considerations
In order to get accurate results from this new tool, the user must pay particular attention to details, according to Samuel Masket, MD, a refractive and cataract surgeon in Los Angeles.
He noted some of these details: “For testing the aphakic refraction, the incision must be sealed but not overhydrated, the central cornea clear, IOP set to physiologic levels (as measured with a tonometer), external pressure from the speculum or drapes eliminated, and, to my sense, all OVD removed. The latter is the subject of a present investigation.”
Dr. Yoo added that the surgeon must also make sure there is no eye tilt during measurement.
Ultimately, said Dr. Masket, “All of these maneuvers add time but contribute to the concept of ‘premium’ cataract surgery. In my view, this tool and similar devices are not for surgeons in a hurry, but for those who are particular about outcomes.”
The Economics of ORA
The ORA device costs about $55,000 (by comparison, this is approximately one-tenth the cost of a femtosecond laser). The number of WaveTec intraoperative aberrometry devices installed throughout the United States has increased fivefold since the end of 2011, Mr. Mahdavi reported.3 He attributed this growth to WaveTec’s 2010 decision to eliminate the peruse “click fee” of $150. Instead, users pay the company a flat $3,000 per month for unlimited use (in addition to the initial purchase cost).
“It removed a huge barrier to entry,” Mr. Mahdavi said. “When they were charging a click fee, that sometimes forced the doctor to make a financial decision instead of a clinical decision. They’ve removed that barrier now, and doctors who have the device are able to use it all they want. It’s like an allyoucaneat buffet as opposed to á la carte.”
Clinical Results Thus Far
Over the last decade, Dr. Ianchulev and other researchers have published and presented results from several clinical studies of intraoperative wavefront aberrometry during cataract surgery. (These studies were performed with the earlier ORange device.) The results showed promise, particularly in eyes that were post-refractive surgery or at the extreme of the axial length spectrum.4-11
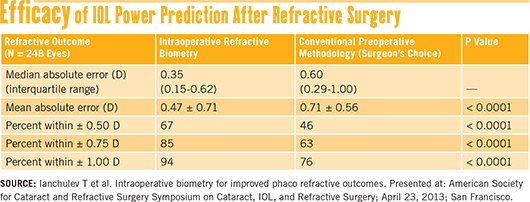
Recent results. More recently, a prospective multicenter study reported on 248 eyes that had prior myopic LASIK or PRK. The results showed that, in these patients, intraoperative biometry led to significantly more predictable refractive outcomes than did conventional preoperative measurements.12
Ophthalmologists who participated in the trial used their normal preop planning methods to select the IOL power for their patients. After performing phacoemulsification, the surgeons did an aphakic refraction with the ORA aberrometer, then compared this result to the preop choice of IOL power.
In 68 percent of the cases, the surgeon decided to alter the preop plan based on the intraoperative refraction, leading to statistically significant differences (p < 0.0001) in postoperative refractive error between the two groups. See their results in the table “Efficacy of IOL Power Prediction After Refractive Surgery” above.
A “compulsive” surgeon’s case series. Dr. Masket reported that a retrospective analysis of more than 150 cataract surgeries in his practice convinced him that ORA improves outcomes, especially in post-refractive surgery eyes.
In a group of best-case cataract patients (135 eyes) who had ORA-assisted cataract surgery, the spherical component of their mean absolute error was within ±0.50 D of the target refraction in 94 percent of these eyes.
Dr. Masket said the case reviews showed that when preoperative IOL calculations and the intraoperative refraction disagreed, he had chosen the ORA refraction 42.7 percent of the time.
“I’m quite compulsive about IOL power selection. I run five formulae on the IOLMaster and four with the Lenstar in each case. So if I change the IOL power based upon the intraoperative ORA readings—then this is worth something,” he said.
Dr. Masket and colleague Nicole Fram, MD, in a separate study of post–laser vision correction eyes requiring cataract surgery, found that with the ORA device, 48 percent of eyes were within ±0.25 D (spherical equivalent) of the target, and 72 percent were within 0.5 D (spherical equivalent); these results compare very favorably to other methods.13
__________________________
1 Ianchulev T et al. J Cataract Refract Surg. 2005;31(8):1530-1536.
2 Koch DD et al. J Cataract Refract Surg. 2012;38(12):2080-2087.
3 Mahdavi S. Impact of ORA on refractive cataract surgery and the premium channel offering. 2013. Accessed July 5, 2013.
4 Chen M. Clin Ophthalmol. 2012;6:397-401.
5 Chen M. Clin Ophthalmol. 2011;5:197-199.
6 Wong AC et al. Ophthalmology. 2010;117(4):711-716.
7 Patwardhan SD et al. J Cataract Refract Surg. 2009;35(7):1190-1192.
8 Sheppard AL et al. Ophthalmic Physiol Opt. 2008;28(6):568-576.
9 Leccisotti A. Graefes Arch Clin Exp Ophthalmol. 2008;246(5):729-733.
10 Leccisotti A. J Refract Surg. 2007;23(9):931-934.
11 Mackool RJ et al. J Cataract Refract Surg. 2006;32(3):435-437.
12 Ianchulev T et al. Intraoperative biometry for improved phaco refractive outcomes. ASCRS Symposium on Cataract, IOL, and Refractive Surgery; April 23, 2013; San Francisco.
13 Masket S, Fram NR. Achieving targeted refractive outcomes in cataract surgery with intraoperative wavefront aberrometer; and Comparison of IOL power calculations in post-LASIK eyes having cataract surgery using multiple formulas, OCT, and intraoperative aberrometry. ASCRS Symposium on Cataract, IOL, and Refractive Surgery; April 23, 2013; San Francisco.
Meet the Experts
David F. Chang, MD Cataract surgeon in practice in Los Altos, Calif., and clinical professor at University of California, San Francisco. Financial disclosure: Is a consultant for Abbott Medical Optics, Clarity, and LensAR; is a lecturer for Allergan; and has equity ownership in Calhoun Vision, Clarity, LensAR, RevitalVision, and Versant Ventures.
Tsontcho “Sean” Ianchulev, MD, MPH Chief medical officer for Transcend Medical; clinical associate professor of ophthalmology, University of California, San Francisco; partner in Tullis Health Ventures; chairman and founder of the KeepYourSight Foundation. Financial disclosure: Holds the original patents on intraoperative refractive biometry; has equity interest in, and is a member of the scientific advisory board for, WaveTec.
Shareef Mahdavi President of SM2 Strategic. Financial disclosure: Mr. Mahdavi’s firm provides consulting services to the medical device industry, including WaveTec.
Samuel Masket, MD Founding partner of Advanced Vision Care and clinical professor of ophthalmology at Jules Stein Eye Institute, Los Angeles. Financial disclosure: Is a consultant for Alcon, Haag-Streit, Ocular Therapeutix, and Power Vision; is a lecturer for Alcon, Bausch + Lomb, and Carl Zeiss Meditec; and has received grant support from Accutome.
Steven I. Rosenfeld, MD Cornea, refractive, and cataract surgeon at Delray Eye Associates, in Delray Beach, Fla., and voluntary professor of ophthalmology at Bascom Palmer Eye Institute. Financial disclosure: None.
Sonia H. Yoo, MD Professor of ophthalmology at the Bascom Palmer Eye Institute. Financial disclosure: Consults for Alcon, Bausch + Lomb, Carl Zeiss Meditec, OptiMedica, and Transcend Medical; has received research funding from Allergan and Genentech.
|