Download PDF
In 1994, the advent of intravenous chemotherapy for retinoblastoma revolutionized the treatment of this pediatric cancer—improving survival rates to more than 95 percent in developed nations and greatly decreasing the need for enucleation. Compared with radiation treatment, complications such as secondary cancers and the risk of metastasis and pinealoblastoma in the brain are reduced.1
But even though intravenous chemotherapy has proved to be more effective than earlier treatment modalities, it comes with its own set of problems, including the risk of neutropenia, infection, and hearing loss. Scientists have also reported cases of secondary leukemia in children who received intravenous chemotherapy for retinoblastoma.2
In the last seven years, however, two new targeted therapies have made it possible to effectively treat even advanced retinoblastoma without enucleation. Intra-arterial and intravitreal chemotherapy can be more potent than intravenous therapy and can cause dramatic regression of tumors in advanced retinoblastoma. In particular, intravitreal chemo has demonstrated effectiveness against vitreous seeding, a condition that is otherwise difficult to treat. Although these therapies have fewer systemic side effects than intravenous chemotherapy, they carry a number of ophthalmic risks that must be balanced against their treatment benefits.
|
Chemotherapy Injection
|
 |
|
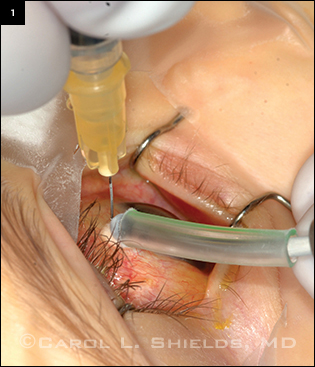
This retinoblastoma patient is being treated with melphalan injected into the vitreous through the pars plana. Cryotherapy is applied as the needle is withdrawn to kill any tumor cells that escape through the needle track.
|
Intra-arterial Chemotherapy
Powerful drug delivery. “With intraarterial chemotherapy, you can get a more powerful dose of medication delivery right to the eye,” said Carol L. Shields, MD, at Wills Eye Institute. “This therapy has enabled us to provide a whole new level of tumor control for patients with advanced disease.”
Intra-arterial chemotherapy was developed and has been used in Japan since the late 1980s, and a somewhat different technique was adopted in the United States in 2006. In the U.S. treatment regimen, the drug is injected through a catheter inserted into the groin and then delivered to the eye’s proximal portion of the ophthalmic artery under fluoroscopic guidance. Although other drugs have been used, the powerful chemotherapy agent melphalan is often the medication of choice for intra-arterial chemotherapy, according to Dr. Shields.
Results. With intravenous chemotherapy, only 48 percent of patients with advanced retinoblastomas (group D eyes) achieve a reduction in their tumors; in contrast, intra-arterial chemotherapy can produce up to 100 percent cancer control in these advanced cancers when used as a primary therapy. As a secondary treatment, it can preserve the eye and provide effective cancer control in 58 percent of retinoblastoma cases, according to recent studies.1
“Intra-arterial chemotherapy has really improved care for retinoblastoma children, and more children than ever are having their eyes saved with this treatment,” Dr. Shields said.
Unanswered questions about intraarterial chemo. Nevertheless, intra-arterial chemotherapy for retinoblastoma is not without controversy. This method has not yet been tested in large prospective trials, while intravenous chemotherapy has been evaluated in several large multicenter studies.
“We need to do prospective trials to show whether or not this therapy is better than others, and if it is better, why? Different agents and methods of administering intra-arterial chemotherapy are now being used around the world, but prospective trials would standardize the protocol,” said Patricia Chévez-Barrios, MD, at the Retinoblastoma Center of Houston. “The point of a prospective trial would be to find out which retinoblastoma patients benefit most from intra-arterial chemotherapy and which patients should be selected for this treatment.”
The Children’s Oncology Group is now planning and seeking funding for a multicenter prospective trial of intraarterial chemotherapy. “There really isn’t a standardized national protocol in terms of dosing and approach,” said Dan S. Gombos, MD, at the M.D. Anderson Cancer Center.
Dr. Gombos noted that melphalan, the drug most often used in intraarterial chemotherapy, is far more toxic than the agents used for intravenous chemotherapy. Some case reports of ophthalmic artery occlusion after intra-arterial chemotherapy have also raised concerns. In addition, the use of fluoroscopy exposes children to radiation, and the exposure may be greater when the procedure is performed by a less-experienced radiologist, he said.
Choosing between therapies. Dr. Gombos acknowledged that whether a patient gets intravenous or intraarterial chemotherapy often depends on the individual medical center. “Some centers are very strong advocates for intra-arterial chemotherapy and are very good at it, while others prefer intravenous chemotherapy. The field of retinoblastoma is still very much in transition, and we know that each type of treatment has its advantages and disadvantages,” he said.
According to Dr. Shields, intra-arterial chemotherapy is often most effective as a primary treatment for those with unilateral sporadic retinoblastoma. It is also used when other treatments have failed. In contrast, bilateral retinoblastoma is most often managed with intravenous chemotherapy because it is a more difficult disease to treat and control. Advanced-stage retinoblastoma may be treated with a combination of intravenous and intra-arterial chemotherapy or enucleation, she noted.
For further information about intra-arterial chemotherapy, see Ophthalmic Pearls in the July 2013 EyeNet.
Intravitreal Chemotherapy
Treatment for vitreous seeding. When vitreous seeding occurs in retinoblastoma, the outlook for patients can be bleak, and many undergo enucleation; systemic chemotherapy and radiation have also been used. However, it’s difficult for chemotherapy to reach and destroy cancer in the vitreous because it is free of blood vessels. Laser rays will pass through the translucent cancerous seeds in the vitreous, producing no effect. “The only treatment other than enucleation with any history of curing vitreous seeds—but is successful in only 50 percent of cases—is radiation,” said Dr. Chévez-Barrios. But external radiation is hardly optimal because of the mutations and secondary cancers that can result, she added. Now, a new treatment—intravitreal (IVT) chemotherapy—may provide a more effective and safer alternative, although research on this modality is in its infancy.
Does IVT promote the spread of cancer? IVT chemotherapy requires injecting medication directly into the eye, and there has been long-standing concern that creating needle or surgical tracks in an eye with cancer allows the disease to spread. For example, vitrectomies performed in eyes with unsuspected retinoblastoma may allow the cancer to spread outside the eye when the surgeon withdraws the surgical instruments. According to Dr. Chévez-Barrios, most patients with unsuspected retinoblastoma who undergo vitrectomies develop metastasis, and many have died of the disease.3
Successes with IVT. Such fears may be countered by research performed in the 1980s in Japan and the early 2000s in the United States showing that intravitreal injections could be used successfully to treat retinoblastoma without promoting extraocular extension or metastasis from the injection site. In the first prospective pilot clinical trial for the treatment of retinoblastoma vitreous seeds using suicide gene therapy—which was also the first trial of gene therapy applied to the eye—Dr. Chévez-Barrios and colleagues demonstrated that, with use of a carefully controlled technique, no needle-track seeding occurred.4
Dr. Shields said that she has used IVT chemotherapy to treat persistent vitreous seeds and has had remarkable success with a dose of 20 to 30 µg of melphalan. “Intravitreal chemotherapy is exciting and is a real advance in treating vitreous seeds in children with retinoblastoma,” she said. “We have seen no systemic side effects after experiences with 50 injections in children with vitreous seeding from retinoblastoma. In addition, the ocular side effects are minimal, and the retina tolerates the treatment very well.”
In a study published in 2012 of 12 patients with vitreous seeding and retinoblastoma, Dr. Shields, in collaboration with researchers in Iran, found that an IVT dose of 10 µg of melphalan achieved control of vitreous seeds in three of seven cases after six months. In four patients who received a 50-µg dose, the tumor control rate was 100 percent, but complications such as cataract, vitreous hemorrhage, and severe hypotony occurred.5 “With a 20- to 30-µg dose we now achieve good control of the vitreous seeds but with very few complications,” Dr. Shields said.
IVT chemo caveat: for experts only. Still, not everyone is convinced that IVT chemotherapy is truly effective or safe. “Intravitreal chemotherapy is a very hot topic right now,” said Dr. Gombos. While acknowledging that recently published scientific papers have shown impressive control of vitreous seeding with minimal complications, Dr. Gombos has concerns about its widespread application.
“Any ophthalmologist or retina specialist can inject IVT chemotherapy because it doesn’t involve a lot of hardware or interventional radiology. The experts who now use this technique know what to do to minimize risks,” he said. However, he fears that in less-experienced hands, the procedure might expose a child to the risk of tumor development outside the eye.
Retinoblastoma is a highly complex cancer that is best managed by those with experience in approaching the disease in a multidisciplinary fashion, according to Dr. Gombos. “I’m cautious about treating a child with intravitreal chemotherapy, but, that said, an appropriately selected patient might benefit from this treatment.” IVT chemotherapy may be a safe and effective treatment for vitreous seeding, but more research needs to be done before it can be used widely, he said.
A Model Protocol for IVT Chemo
Researchers in Switzerland have had considerable success administering IVT chemotherapy according to a treatment protocol they developed.6 They administer up to eight injections of 20 to 30 µg of melphalan in eyes with vitreous seeding. In a retrospective study, Francis L. Munier, MD, at the Jules Gonin Eye Hospital in Lausanne, Switzerland, reported resolution of vitreous seeding and retinoblastoma in 87 percent of heavily pretreated patients 22 months after IVT therapy.7 In all but two patients, however, local treatments, including ruthenium plaques, cryotherapy, and thermotherapy, were also needed to control the cancer that was the source of the vitreous seeds as well as subretinal vitreous seeds. Three patients were enucleated for reasons unrelated to the IVT chemotherapy, but no patients developed metastatic disease. Complications were limited to the site of the injection and included salt-and-pepper retinopathy in 43 percent of patients as well as transient vitreous hemorrhage in two patients.7
Dr. Munier has now administered more than 300 injections of this therapy in 60 patients. “Achieving 80 to 90 percent success treating vitreous seeding in retinoblastoma patients is just unprecedented,” he said. “Intravitreal chemotherapy now looks like an effective way to beat vitreous seeds, a major cause of retinoblastoma therapy failure.”
He emphasized, however, that IVT chemotherapy is a salvage therapy to be used in cases of recalcitrant or recurrent vitreous seeding. It should not be used as a primary therapy.
Dr. Munier follows a strict protocol to mitigate the risk of cancer dissemination as a result of IVT injection. Before the procedure, he assesses each eye with ultrasound biomicroscopy to determine if there is a safe meridian in the eye to inject with a needle. He applies five criteria in determining safety: 1) the presence of clear media; 2) the absence of invasion of the anterior and posterior chamber; 3) the absence of tumor at the entry site; 4) the absence of vitreous seeds at the entry site; 5) the absence of retinal detachment at the entry site.6
To avoid possible reflux due to increased intraocular pressure from the injection, Dr. Munier first withdraws the same volume of vitreous as will be administered in the chemotherapy solution. The injection is administered through a 32-gauge needle to minimize the diameter of the scleral opening, which is only about 10 µm. Finally, to block the egress of cancer cells after the chemotherapy is injected, he applies three cycles of freeze and thaw cryotherapy to the site of the injection when the needle is withdrawn (Fig. 1). He said that the use of this freezing technique will likely destroy any cancer cells that escape.
The safety of this protocol was studied in a group of 30 consecutive retinoblastoma patients who received a total of 135 IVT chemotherapy injections. No case of extraocular or systemic spread of tumor was seen during 13.5 months of follow-up (range, 1-66 months).6
Although the potential risks of IVT chemotherapy include cataract, hemorrhage, retinal detachment, and infection, said Dr. Munier, these problems can generally be avoided through use of appropriate techniques. For example, placing the injection 3.5 mm from the limbus reduces the risk of lens damage.
Dr. Munier is spearheading a prospective multicenter clinical trial, which is currently recruiting patients in several countries. “Intravitreal chemotherapy now seems to be a very efficient approach to treating vitreous seeds. This therapy helps improve prognosis in retinoblastoma with vitreous seeds, and it greatly increases the probability that enucleation can be avoided,” he said.
___________________________
1 Shields CL et al. Eye (Lond). 2013;27(2):253-264.
2 Jabour P et al. J Neurosurg Pediatr. 2012;10(3):175-181.
3 Shields CL et al. Ophthalmology. 2000;107(12):2250-2255.
4 Chévez-Barrios P et al. J Clin Oncol. 2005;23(31):7927-7935.
5 Ghassemi F, Shields CL. Arch Ophthalmol. 2012;130(10):1268-1271.
6 Munier FL et al. Br J Ophthalmol. 2012;96(8):1084-1087.
7 Munier FL et al. Br J Ophthalmol. 2012;96(8):1078-1083.
___________________________
Patricia Chévez-Barrios, MD, is codirector of the Retinoblastoma Center of Houston and director of the ocular pathology research laboratory at Methodist Hospital in Houston. Financial disclosure: None.
Dan S. Gombos, MD, is associate professor and chief of ophthalmology in the head and neck surgery department at M.D. Anderson Cancer Center and codirector of the Retinoblastoma Center of Houston with a joint appointment at Baylor College of Medicine in Houston. Financial disclosure: None.
Francis L. Munier, MD, is professor and head of the retinoblastoma clinic at the Jules Gonin Eye Hospital in Lausanne, Switzerland. Financial disclosure: None.
Carol L. Shields, MD, is associate director of ocular oncology at the Wills Eye Institute and professor of ophthalmology at Thomas Jefferson University Hospital, in Philadelphia. Financial disclosure: None.
More at the Meeting
For an overview of recent advances in the diagnosis, imaging, staging, and treatment of retinoblastoma, plan to attend instruction course 579, “Retinoblastoma 2013: They Live and See!” (Nov. 19, 12:45-3 p.m.).
|