By Arun D. Singh, MD, Jorge J. Echegaray, MD, and Jaqueline Davanzo, BSN
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Iris tumors and their simulating conditions are amenable to excisional biopsy1,2 depending upon the size, location, and suspected clinical diagnosis.3-6 Where indicated, surgical excision for localized iris melanoma has the advantage of providing diagnosis, prognosis, and treatment with a single surgical procedure.7 The surgical excision can be limited to the iris (iridectomy) or ciliary body (cyclectomy), or it may involve a combination of both tissues (iridocyclectomy).![]()
 |
|
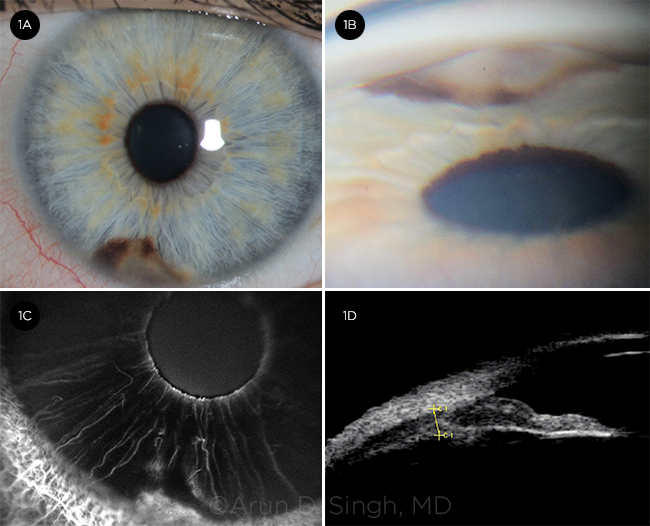
BEFORE THE PROCEDURE. A 37-year-old Caucasian woman had an iris lesion since age 16, and her optometrist noted that it had enlarged. Visual acuity was 20/20 in both eyes with normal intraocular pressures bilaterally. Slit-lamp examination of the left eye disclosed mild corectopia and a pigmented iris lesion inferiorly measuring 4 mm x 3 mm x 2 mm (1A). On gonioscopy, the central aspect of the lesion had an amelanotic nodular component (1B). Anterior segment fluorescein angiogram revealed hyperfluorescence consistent with intrinsic vascularity (1C). Ultrasound biomicroscopy showed absence of ciliary body extension (1D).
|
Surgical Steps
The surgical technique of iridocyclectomy can be considered as a sequence of steps, including those below. (See accompanying video below.)
1. Conjunctival flap. The conjunctival flap extends at least 1 clock hour beyond the limits of the proposed outer scleral flap. In general, a fornix-based conjunctival flap is preferred over a limbus-based approach, as it offers a better view of the inner scleral/corneal incisions. A conjunctival flap may not be required if the incision to access the anterior chamber is at the limbus, such as during iridectomy.1-3, 8-10
2. Outer scleral flap. The size and location of the outer scleral flap is determined by the size and location of the tumor as confirmed by preoperative ultrasound biomicroscopy and intraoperative transillumination. The flap is about 2 mm beyond the lateral and peripheral tumor margins. As an example, for a 4-mm-wide tumor, the ideal outer scleral flap will be 8 mm wide. The outer scleral flap is outlined on the sclera with a fine-tipped marking pen after the sclera is dried and bleeders are cauterized.
The depth is about 80%-90% of scleral thickness (1 mm near the limbus). The desired depth of incision can be achieved by using a diamond blade. Once the plane of dissection of desired depth is created, the flap is reflected with a crescent blade. Optimal flap thickness is critical because a thin flap poses problems in achieving watertight wound closure while a thick flap will expose underlying uvea or tumor with resultant bleeding.
3. Inner scleral/corneal incision. The limits of the inner scleral flap should be constructed 1 mm smaller than the outer scleral flap (and 1 mm beyond the lateral and peripheral tumor margins). This 1-mm strip of overlap facilitates watertight wound closure.
The initial incision into the inner scleral flap is made with an upward cut using a microincision blade. The incision is then extended using fine scissors, which exposes the underlying ciliary body. The radial margins are extended up to the limbus and then circumferentially along the limbus by entering the anterior chamber with left and right cutting corneal scissors for about 2 mm beyond the visible tumor margin.
The inner scleral incision also is extended along the limbus, thereby freeing the inner scleral flap from the cornea. The anterior chamber can be stabilized with the use of viscoelastic at any stage during the procedure.
4. Uveal incisions. Once the inner scleral incisions are made as described above, the underlying ciliary body becomes visible. Cautery is applied to the ciliary body before it is incised.
The uveal incision is initiated from the iris by creating a small iridotomy 1 mm beyond the iris margin of the tumor with the possibility of sparing iris sphincter muscle. The iridotomy is converted to a peripheral iridectomy by extending the iridotomy toward the limbus incising a triangular portion of the peripheral iris and staying 1 mm beyond the visible tumor margin.
If the tumor is close to the pupillary margin or involves the pupil, then the incision is initiated from the pupil, creating a sector iridectomy.
The cornea is lifted gently by the assistant to provide adequate exposure, thereby avoiding contact with the corneal endothelium and the lens.
The iris incisions are extended into the ciliary body corresponding to the limits of the inner scleral flap. Bleeding during this part of the surgery can be controlled by careful application of cautery or topical application of epinephrine (1 in 100,000), phenylephrine (2.5%), or thrombin (5,000 IU/mL).
Any uveal bulge or vitreous upthrust can be relieved by releasing any inadvertent pressure on the globe or by aspirating 0.1 mL of the vitreous through the pars plana (4 mm behind the limbus, using a 25-gauge short needle attached to a tuberculin syringe).
5. Pupilloplasty. In cases of large peripheral iridectomy or sector iridectomy (smaller than 1 clock hour), pupilloplasty is attempted with a 10-0 nylon suture using the modified Siepser technique.11
6. Wound closure. Meticulous watertight closure is achieved with 8-0 and 10-0 nylon sutures. Overlapping edges between the outer and inner scleral incisions facilitate good closure. At the end of the procedure, the anterior chamber should be of normal depth. Small residual hyphema can be left in the anterior chamber as it tends to absorb spontaneously without secondary complications.
 |
|
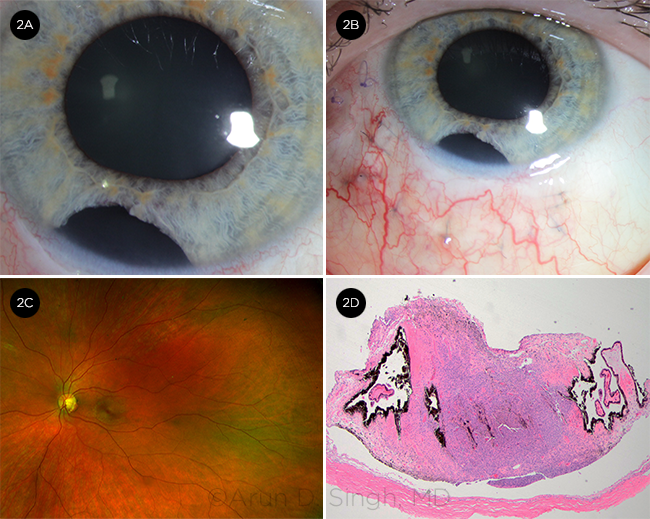
AFTER THE PROCEDURE. Our clinical impression was of an iris melanoma. After an explanation of the risks and benefits of the procedure, the patient agreed to proceed with our recommendation of iridocyclectomy. Two weeks postoperatively, the cornea was clear without hyphema (2A). The surgical site was well healed (2B). The posterior segment was normal without hemorrhage or retinal detachment (2C). Histopathology confirmed the presence of iris melanoma, of a mixed spindle cell and epithelioid type. Mitotic activity was inconspicuous. The tumor cells were MelanA and HMB45 positive (not shown). Central bluish-colored tumor with thin scleral flap along the base was flanked by normal ciliary processes on either side (2D). (Hematoxylin and Eosin, 40 x.)
|
Discussion
The first reported case of iridocyclectomy is attributed to Zirm (1911)12 and the early experience of iridocyclectomy from 1911-1970 has been reviewed elsewhere by Vail.13 The technique reported herein has evolved over the years based upon the work of Stallard14 and others.
The optimal outcome following iridocyclectomy requires careful selection of cases, familiarity with surgical steps, awareness of potential complications, and appropriate postoperative management. The surgical technique can be modified to perform iridectomy or cyclectomy alone, although iridocyclectomy is performed most often. Repair of a large corneoscleral incision has the potential for wound leak, hypotony, hyphema or hemorrhage, cataract, corneal edema, and astigmatism with visual recovery requiring up to 6 weeks or longer,10,15 reminiscent of recovery after extracapsular cataract surgery.16
As uveal melanoma may extend into the overlying sclera,17 creation of a partial-thickness scleral flap allows removal of melanoma cells that may be present in the inner sclera. This technique also obviates the need for a full wall resection, which requires use of graft material for closure.18 In addition, a partial-thickness scleral flap allows watertight closure of the wound, minimizing the risk of hypotony. Excessive resection of ciliary body (more than half) is not recommended, as it may lead to chronic hypotony.19,20
The long-term functional results after iridocyclectomy are good, and complications and recurrences are rare.20-22 Intraoperative hemorrhage is the most frequent complication, which can be minimized, although not completely avoided, with the use of hypotensive anesthesia, cautery, and diathermy of the uvea. Topical thrombin (5,000 IU/mL) is easy to apply and effective.
Potential for incomplete excision, lack of definite assessment of tumor margins,22 and concerns for delayed recurrence20 are important considerations before recommending iridocyclectomy. Such concerns are particularly valid for larger, posteriorly located tumors (choroidectomy).23 Adjuvant use of radiation is associated with a lower risk of recurrence.23
___________________________
1 Pe’er J, Frenkel S. Br J Ophthalmol. 2011;95(10):1474-1476.
2 Klauber S et al. Acta Ophthalmol. 2012;90(2):122-126.
3 Reese AB, Cleasby GW. Am J Ophthalmol. 1959;47(5, Part 2):118-125.
4 Khan S et al. Arch Ophthalmol. 2012;130(1):57-64.
5 Shields CL et al. Ophthalmology. 2012;119(2):407-414.
6 Shields CL et al. Ophthalmology. 2001;108(1):172-178.
7 Damato B, Groenewald C in Clinical Ophthalmic Oncology, eds AD Singh et al. Philadelphia: Saunders Elsevier; 2007:259-266.
8 Rones B, Zimmerman LE. Arch Ophthalmol. 1958;60(2):193-205.
9 Batioglu F, Gunalp I. Jpn J Ophthalmol. 1998;42(4):281-285.
10 Arentsen JJ, Green WR. Ophthalmic Surg. 1975;6(2):23-37.
11 Osher RH et al. J Cataract Refract Surg. 2005;31(6):1098-1100.
12 Zirm EK. Arch Augenheilk. 1911;69:233.
13 Vail DT. Am J Ophthalmol. 1971;71(1 Pt 2):161-168.
14 Stallard HB. Br J Ophthalmol. 1966;50(11):656-659.
15 Cardine S et al. J Fr Ophtalmol. 2003;26(1):31-37.
16 Alio JL, Fine IH. Minimizing Incisions and Maximizing Outcomes in Cataract Surgery. New York: Springer; 2010.
17 Albert DM et al; COMS Research Group. Am J Ophthalmol. 1998;125(6):745-766.
18 Peyman GA et al. Ophthalmic Surg. 1986;17(12):822-829.
19 Barrada A et al. Retina. 1984;4(2):119-122.
20 Memmen JE, McLean IW. Ophthalmology. 1990;97(4):429-432.
21 Daubner D et al. Klin Monbl Augenheilkd. 2008;225(12):1045-1050.
22 Forrest AW et al. Ophthalmology. 1978;85(12):1237-1249.
23 Damato BE et al. Br J Ophthalmol. 1996;80(2):102-108.
___________________________
Dr. Singh is director of Ophthalmic Oncology, Dr. Echegaray is clinical fellow, and Ms. Davanzo is a nurse; all are at the Department of Ophthalmic Oncology, Cole Eye Institute, Cleveland Clinic, Cleveland, Ohio. Relevant financial disclosures: Dr. Singh—Aura Biosciences: C; Castle Biosciences: C; Iconic Therapeutics: C; IsoAid Therapeutics: C. In addition, Dr. Singh has a pending patent “Risk calculator for vision loss following brachytherapy.”
See the disclosure key at www.aao.org/eyenet/disclosures.
Acknowledgments
Werner Wackernagle, MD, Rubens Belfort MD, Virginia Torres, MD, Mary Beth Aronow, MD, Carlos Medina, MD, Yahya Zahrani, MD, Hassan Aziz, MD, Sean Platt, MD, Claudine Bellerive, MD, Annapurna Singh, MD, Leslie Lane, CST, Jacqueline Davanzo, BSN, and Dina Petrich, COT, for insightful comments related to the surgical technique and for care of the patients. Crain Smith for video recording.
|