Download PDF
Only a handful of ophthalmic surgeons in the United States perform iris reconstruction using prosthetic devices, but those who do are passionate about it. They would have to be, to endure the red tape, regulatory setbacks, and hours of uncompensated time spent filing documents to obtain permission to implant the devices. Because there are no FDA-approved iris prostheses, permission is granted to U.S. surgeons through investigational or compassionate use exemptions only. In contrast, such devices have been available in Europe for more than 15 years. The major manufacturers are Morcher (Stuttgart, Germany), Ophtec (Groningen, the Netherlands), and HumanOptics (Erlangen, Germany).
Despite impediments to the use of iris prostheses in the United States, including the glacial pace of progress in obtaining device approvals, the field is moving forward. In fact, a much-anticipated U.S. clinical trial of the HumanOptics CustomFlex Artificial Iris is expected to launch soon.
Three U.S. ophthalmologists who have been implanting iris prostheses discuss their experience with these devices, recent developments, and the regulatory path still to be traversed.
Barriers vs. Benefits
Kevin M. Miller, MD, professor of clinical ophthalmology at the Jules Stein Eye Institute in Los Angeles, estimates that only 10 to 15 surgeons in the United States perform artificial iris implantation. “Those of us who do this work go patient by patient, filing for compassionate use device exemptions, or CUDEs,” he said. “It takes 11 different documents to obtain a single FDA CUDE, and you also have to obtain approval from a local institutional review board. The local approval is an entirely different process that requires additional pages of filings. This is all very labor intensive, requiring hours and hours of work with no compensation for the surgeon.
“But these patients are miserable. You feel bad for them, and they have nowhere else to go.” Apart from cosmetic issues, patients with congenital aniridia or traumatic iris loss may have severe problems with visual function, including disabling light sensitivity, glare, and reduced visual acuity.
|
Aniridia Solution
|
 |
|
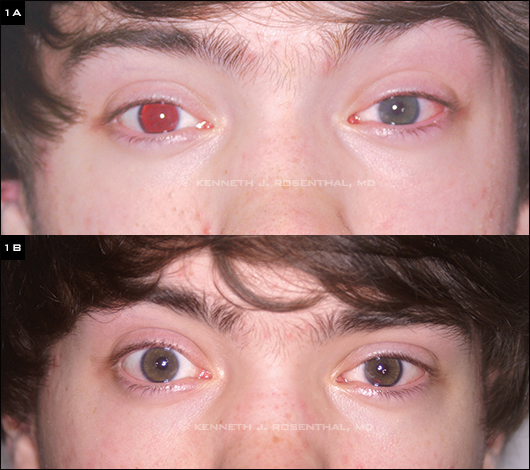
(1A) 17-year-old with congenital bilateral aniridia after receiving HumanOptics iris implant in left eye and (1B) both eyes. Preimplant BCVA of 20/100 OU improved to 20/30 UCVA OU after surgery, likely due to creation of normal-sized pupils.
|
Morcher Devices
Although Morcher iris devices have a long history of use in Europe, Dr. Miller is the only surgeon in the United States who is currently permitted to implant these prostheses. He received an investigational device exemption (IDE) from the FDA about 10 years ago, and when the agency stopped issuing CUDEs for the Morcher implants three years ago, it allowed Dr. Miller to continue using them. Although the agency did not explain its discontinuation of the CUDEs, he suspects that lack of requested follow-up data from some physicians may have played a role.
Models in U.S. study. Dr. Miller, a coauthor of a 2008 clinical study report on several Morcher models,1 said that only one change has been made in the last five years in the product line he is studying. The devices included in his IDE are the 96F partial aniridia ring, the 50F aniridia ring, and the 67B aniridia implant. The 50F is a redesign of the 50D (also known as the Rasch-Rosenthal design).
“The 96F is a small modification of a capsular tension ring—basically a CTR with a black paddle that covers 90 degrees of arc,” Dr. Miller said.
“The original 50D had eight occluder paddles, and if you placed two of these rings and overlapped them perfectly, you would have an artificial iris with a 4-mm aperture. But the alignment had to be absolutely perfect, and on the operating table it was difficult to align the rings. The next day little slits may have formed between the paddles, letting the light pass through. In the 50F redesign, the paddles were made larger.”
The 67B is a lens implant with a surrounding black artificial iris. According to Dr. Miller, the 67B has the smallest pupil at 3 mm but functions better than the other devices with much larger pupils.
Dr. Miller has implanted more than 60 Morcher devices so far, and he expects to reach the 70 implants required for review by the FDA sometime this year. “We will follow the patients for one year and then send our data to the FDA,” he said. “But I have no road map for how or when or if these devices will be on the market.”
 |
|
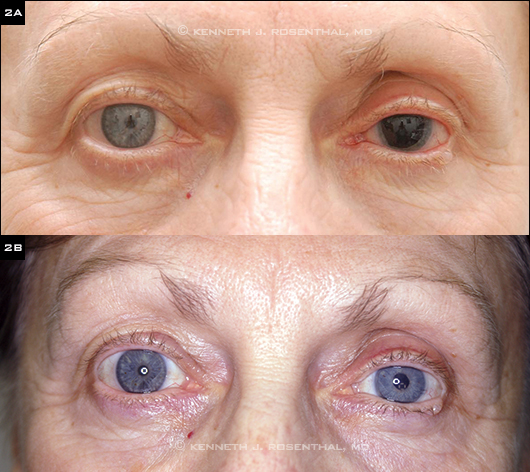
TRAUMA REPAIR. (2A) 62-year-old woman who was struck in the left eye by a tennis ball suffered traumatic mydriasis, cataract, and retinal detachment.Preoperative view after repair of retinal detachment and cataract extraction. (2B) One day after secondary IOL implantation, using glued IOL technique, and implantation of custom matched HumanOptics iris prosthesis.
|
Ophtec 311 Iris Reconstruction Lens
The Ophtec 311 is designed for patients who are aniridic and either aphakic or require cataract surgery. A clinical study of the Ophtec 311 has been ongoing for 10 years. “It is anticipated that the company will eventually move forward toward obtaining FDA approval for the device,” said Kenneth J. Rosenthal, MD, surgeon-director of Rosenthal Eye and Facial Plastic Surgery in New York City and Great Neck, N.Y., and a principal investigator in the Ophtec trial. He is also an associate professor of ophthalmology at the University of Utah and an attending surgeon at New York Eye and Ear Infirmary. “In the meantime, study investigators continue to have access to these devices.” He added that the Ophtec 311 iris implant is “not cosmetically perfect by any means, but functionally the devices are excellent and represent an appreciable improvement over the original device that Volker Rasch, MD, and I introduced in 1996.” Color choices for these artificial irides are blue, green, and brown.
Study update. The Ophtec study has been modified several times, and multiple substudies have been spun off, Dr. Miller said. “Once the study had accrued the required number of patients, a continuing access study was created so that other patients could be enrolled and followed for one year. But three-year follow-up data from the primary study was available three or four years ago, and to my knowledge, there has been no meeting with the FDA.”
Dr. Miller noted that the patients coming into the trial tend to have other ocular problems in addition to their iris defects. “As a result, we see all kinds of adverse events, including retinal detachments, glaucoma, and poor visual outcomes—but that’s what these eyes have coming in, and we make them a little better. I believe that’s where the FDA has a problem.” He said that the agency considers the Ophtec 311 a lens implant, “and when you compare it against the FDA standards for IOLs in general, it falls short.”
HumanOptics Artificial Iris
Trial in the works. “The real headline in iris prostheses is what we are hoping will be the upcoming clinical trial of the HumanOptics Artificial Iris,” said Dr. Rosenthal, who is principal investigator at the New York Eye and Ear Infirmary study site. The 12-center study is expected to include patients with a congenital, traumatic, or acquired iris
defect that is visually significant due to light sensitivity or other problems.
The trial will be limited to adults, said the U.S. study’s medical monitor, Michael E. Snyder, MD, a surgeon at the Cincinnati Eye Institute and member of its board of directors. “We are currently seeing patients through compassionate use exemptions,” he said, “and we hope to roll some of that data into the FDA submission once we get to that stage.” Dr. Snyder expects the study to recruit about 150 patients.
“We hope to get the trial accrued and completed relatively quickly so that our peers will have an FDA-approved device to use in treating these patients,” Dr. Snyder added.
Device details. The HumanOptics device has been used in Europe for about 10 years, and surgeons in the United States have about 4.5 years of data. Approximately 550 of the devices have been implanted worldwide, and roughly 150 of those are stateside. “Our cohort at the Cincinnati Eye Institute has implanted 105 Human-Optics devices,” Dr. Snyder said. “We hope to have all our study centers up and running quickly so that we can recruit more patients and obtain a wider range of experience with multiple investigators and sites.”
The advantages of this artificial iris device include the relatively small incision size required and superior cosmetic appearance. “Even though the match between the two eyes may not be perfect in every case, from ‘cocktail party distance’ it is very difficult to see any difference between the two eyes,” Dr. Snyder said. “The pseudopupil is also a little smaller than that of some of the other manufactured devices.”
The HumanOptics implant is available in two designs. One is made entirely of silicone impregnated with color granules deep within the silicone matrix. The other includes a polyester mesh embedded in the silicone matrix that holds sutures more tightly without the risk of cheese-wiring.
The device, which does not incorporate a lens component, is placed in front of an IOL. It can be cut to size for placement in the capsular bag. “It has a 3.35-mm central aperture for the pupil and comes manufactured with a 12.8-mm outer diameter,” Dr. Snyder said. “For a normal adult capsular bag, we would typically cut the device to about 9.5 mm, and with a particularly large capsular bag, maybe to 10 mm. With children, we measure the capsular bag after the capsular tension ring has been placed.” Dr. Snyder added that whenever he places a device in the capsular bag, he inserts a CTR to prevent potential contraction of the bag and distortion of the device later on.
Is There a Downside?
Without sufficient clinical trial data, it is difficult to quantitatively assess the possible risks associated with these devices. Moreover, the patients who require iris implants generally have multiple comorbid eye diseases, said Dr. Snyder. “Accordingly, lots of ‘complications’ occur in these patients, though complications due to the device are pretty uncommon.” He has had to reposition a few and is aware of some U.S. cases in which the device was explanted, though he believes “the device was a bystander to other pathology.” Dr. Miller said that he has had “essentially zero issues” with iris prostheses and has explanted only one in more than 10 years—and that was from a severely traumatized eye.
Dr. Miller noted that if the implants are placed in the sulcus rather than the capsular bag, they may have intermittent uveal touch and occasionally cause inflammation. Dr. Rosenthal prefers to suture fixate devices not placed in the bag; he suggests that stabilizing the implant reduces risk of inflammation.
Cosmetic Iris Implants: The Ocular Damage Remains
Eye color is the latest physical attribute to be subject to alteration for cosmetic reasons. Unfortunately, many brown-eyed seekers of blue or green eyes have found themselves with serious, potentially sight-robbing ocular problems. The NewColorIris implant was developed by Kahn Medical Devices in Panama City, Panama, where the surgery was performed. Although the company reportedly is now out of business, ophthalmologists may still see patients who are suffering vision-threatening sequelae related to the device. Unlike the devices discussed in the main story, which replace a missing or severely damaged iris, these are implanted in front of a normal iris.
“In 2009 there was a lot of media frenzy about the NewColorIris,” said Shameema Sikder, MD, assistant professor of ophthalmology at Johns Hopkins and medical director of Wilmer at Bethesda (Md.). “On one of the TV segments promoting the cosmetic implants, a patient who had received the devices said, ‘There are risks with any surgery, but this is totally reversible, and the implants can be taken out.’ Unfortunately, the complications are not reversible, including the development of glaucoma leading to blindness and decompensation of the cornea requiring cornea transplantation.” Uveitis and hyphema also have been reported.
In a 2011 article, Dr. Sikder and her colleagues reported on several potential causes of ocular damage from the cosmetic implants.1 Their paper cited manufacturing defects, surface irregularities, and sharp edges that caused abrasions of the corneal epithelium and iris. In one report, several of the peripheral footplates designed to protect the implant from interacting with the trabecular meshwork were missing, probably from manipulation of the implant prior to surgery. In addition, the NewColorIris had a tendency to contact angle structures directly and to shift over time. Improperly sized implants also tended to vault within the anterior chamber.
“From reviewing the literature, we have seen two categories of patients: the acute cases who present within one month of implantation and those who have a delayed presentation within six months,” Dr. Sikder said. “Most of these patients end up undergoing explantation. Although some patients may regain their preimplant vision, others end up 20/40 or less, sometimes with vision as poor as counting fingers only. In some cases, the endothelial cell counts go down, requiring a corneal transplant for visual rehabilitation.”
___________________________
1 Sikder S et al. Clin Ophthalmol. 2011;5:435-438.
|
The Patient’s Feelings
Dr. Snyder pointed out an often-overlooked and rewarding aspect of giving a patient a cosmetically appealing artificial iris. “Many patients who have iris abnormalities undergo significant changes in their body image, especially those with light-colored eyes,” he said. “They don’t feel as confident in themselves—they don’t feel quite normal. As clinicians we need to give these patients permission to tell us how they feel about that. When I ask patients if the appearance of their eyes bothers them, some will say, ‘Oh, no. It doesn’t bother me.’ But a few moments later, often with a few tears, they will say, ‘Yeah, it bothers me a lot. People are always staring at my eye.’”
The newer iris prostheses may change that. Dr. Snyder relayed the experience of a patient who had suffered severe, disfiguring damage to her eye in a car accident as a young child. Now in her 20s, the young woman recently underwent implantation of a HumanOptics artificial iris.
“She called to tell me that receiving the artificial iris was one of the best things that had ever happened to her,” Dr. Snyder said. “She still calls or sends a note periodically just to remind me and my team of her happy result.”
___________________________
1 Olson MD et al. J Cataract Refract Surg. 2008;34(10):1674-1680.
___________________________
Dr. Miller receives grant support from Alcon, Calhoun Vision, Hoya, and Physical Optics and receives lecture fees from Alcon. Dr. Rosenthal is a consultant for Abbott Medical Optics, Alcon, Bausch + Lomb, Inspire, Ista, Johnson & Johnson, Microsurgical Technologies, and Ophtec; he receives lecture fees and grant support from Abbott Medical Optics and Ophtec. Dr. Sikder is a consultant for Allergan. Dr. Snyder is a consultant for Alcon and Dr. Schmidt Intraocularlinsen and receives lecture fees from Alcon and Haag-Streit.