Download PDF
A review of studies on the natural history of keratoconus has found that children and those with a maximum keratometry (Kmax) steeper than 55 D at presentation have a significantly higher risk of disease progression.1 These patients need careful monitoring and a lower threshold for collagen cross-linking (CXL) to prevent further disease progression, the authors said.
The findings emerged from a sys tematic review of 41 publications. Of these, 23 studies with 12-month outcomes were included in a meta-analysis. “It was surprising how few modern studies have investigated the natural progression of keratoconus,” said Alex C. Ferdi, MD, at the University of Sydney in New South Wales, Australia. “Yet knowledge of the natural history is crucial to understanding progression and hence the need for interventions such as CXL.”
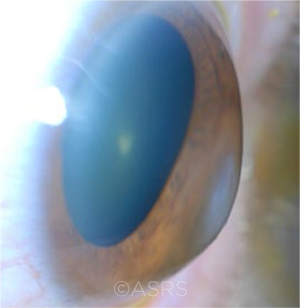 |
EVALUATION. Keratoconus patients who have steeper corneas and those who are younger at presentation may need more frequent follow-up. This image was originally published in the ASRS Retina Image Bank. Jason S. Calhoun. Keratoconus. Retina Image Bank. 2013; Image Number 7634. © The American Society of Retina Specialists.
|
At greatest risk. The results indicate that young patients progress more aggressively than adults; those younger than 17 years were more likely to have more than 1.5 D of Kmax progression at 12 months. With regard to the severe progression noted among all patients with steeper Kmax at initial assessment, those with greater than 55 D Kmax at presentation were likely to progress by at least 1.5 D Kmax at the one-year mark.
In addition, Middle Eastern patients experienced more progression over 12 months than did European and East Asian patients, the researchers found. They called for further studies to clarify the influence of ethnicity on keratoconus progression.
A note on topography. Earlier studies demonstrated that progression was associated with significant changes in visual acuity and refraction.2,3 In contrast, this meta-analysis found no significant progression related to changes in these factors. In addition, the rate of thinnest pachymetry change was not clinically significant. While these are important aspects of progression, they may be less sensitive measures of progression than topography, the researchers suggested.
In the clinic. Dr. Ferdi advised clinicians to consider age and corneal parameters when evaluating the risk of progression and the risks and benefits of CXL. His institute has increased the frequency of follow-up visits for patients with keratoconus who have steeper corneas and are at younger age at presentation. In addition, they now have a lower threshold for recommending CXL in such patients.
Dr. Ferdi also urged clinicians to report patient data to the Save Sight Keratoconus Registry (https://frbresearch.org). “Our study highlighted an urgent need to collect data on keratoconus to add to our knowledge of disease natural history and to understand treatment outcomes and how individual patients respond to CXL,” he said.
—Miriam Karmel
___________________________
1 Ferdi AC et al. Ophthalmology. Published online March 8, 2019.
2 Tuft SJ et al. Ophthalmology. 1994;101(3):439-447.
3 Wagner H et al. Cont Lens Anterior Eye. 2007;30(4):223-232.
___________________________
Relevant financial disclosures—Dr. Ferdi: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Esmaeli None.
Dr. Ferdi Northcote Trust: S.
Dr. Rong None.
Dr. Tam None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review