By Miriam Karmel, Contributing Writer, interviewing Greg Hoffmeyer, Gerard A. Lutty, PhD, Robert F. Mullins, MS, PhD, and Glenn C. Yiu, MD, PhD
Download PDF
The choroid is regarded as something of a black box, obscured as it is by the retinal pigment epithelium (RPE), under which it sits. With recent advances in imaging, however, the lid has been lifted off the box.
Most high-end optical coherence tomography (OCT) systems currently available allow ophthalmologists to see in vivo this highly vascular layer of the eye that lies between the retina and sclera. With these tools, ophthalmologists can begin to understand how the vasculature changes and how that affects disease states, said Gerard A. Lutty, PhD, at the Wilmer Eye Institute in Baltimore.
Seeing the choroid not only makes it more accessible to clinicians but is also changing the management of certain diseases, said Glenn C. Yiu, MD, PhD, at the University of California, Davis. And in terms of research, more attention is being paid to this tissue that supports the photoreceptors with oxygen and nutrients, said Robert F. Mullins, MS, PhD, at the University of Iowa (see “Research Update”).
 |
|
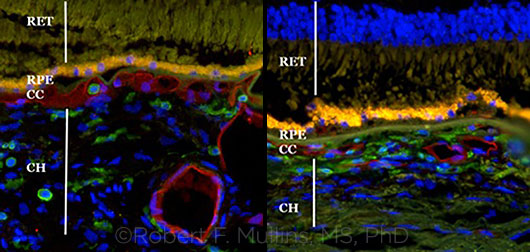
COMPARISON. This pair of images shows a healthy choroid (left) and a degenerated one with drusen (right).
|
OCT-A and EDI-OCT
This latest facet of the imaging revolution is being driven primarily by OCT angiography (OCT-A) and enhanced- depth imaging OCT (EDI-OCT).
OCT-A is an application for spectral- domain (SD-OCT) and swept-source OCT (the latter has yet to be approved in the United States for visualizing the choroid). Unlike EDI-OCT, which gives a cross-sectional view, OCT-A is usually viewed en face, as a bird’s-eye view. It uses superfast scanning to visualize blood flow, and it can focus at different levels to provide information on both retinal and choroidal vessels. “There is tremendous excitement surrounding OCT-A, especially because you do not need to inject dye, so it is much faster [than fluorescein angiography],” Dr. Yiu said. Although OCT-A cannot show dye leakage in the traditional way, vessel details also aren’t affected by leaking dye, giving unprecedented details of small vessels, he added.
High-resolution OCT-A reveals movement of blood cells, which Dr. Lutty said could lead to preventive treatment. “In our work, we have seen that the small vessels in the choroid die in AMD.”1 He added, “You can actually see when these small blood vessels don’t have blood cells in them. If you knew the small blood vessels were not functional [no blood cell movement], you could predict where choroidal neovascularization [CNV] might form, and it might allow you to treat the patient prophylactically.”
EDI-OCT is a software application built into newer SD-OCT machines, said Gregory Hoffmeyer, who is with Carl Zeiss Meditec. It takes advantage of the greater depth and choroidal details of the image that the machine generates, and the cross-sectional view exposes all the layers of the choroid, from the large vessels to the choriocapillaris. Researchers using EDI-OCT have found that some conditions coincide with either abnormal thickening or thinning of the choroid.
Ophthalmologists who want to see the choroid can ask the technician to use the EDI feature, said Mr. Hoffmeyer. However, he cautioned, on some machines, you may lose some resolution in the retina when you click on EDI.
 |
|
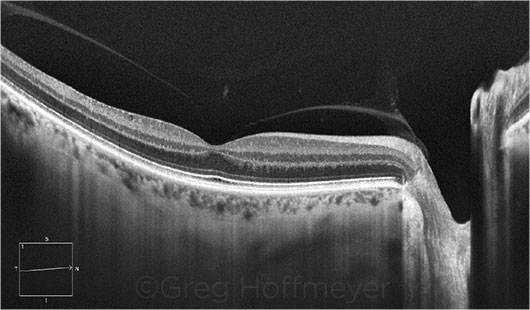
GLAUCOMA SUSPECT. This patient may have early glaucoma. EDI-OCT shows that the choroid is thin, compared with the retinal thickness.
|
Potential Applications
EDI-OCT for pachychoroid diseases. These include a spectrum of conditions with abnormal thickening of the large choroidal vessels. A thickened choroid may arouse suspicion of central serous chorioretinopathy (CSC), Dr. Yiu said. Increased choroidal thickness helps distinguish CSC from other conditions and can help guide treatment. For example, after photodynamic therapy (PDT) for CSC, resolution of subretinal fluid may be associated with choroidal thinning over time.
Another benefit of seeing the choroid involves polypoidal choroidal vasculopathy, which is often treated with anti-VEGF agents and PDT. There is evidence that the anti-VEGF agents can reduce leakage, but PDT may be better at getting the polyps—which extend from the choroid into the subretinal space—to regress, Dr. Yiu said.
OCT-A for neovascular disease. “We used to classify CNV based on leakage patterns on fluorescein angiography,” Dr. Yiu said. Then OCT came along and allowed ophthalmologists to quantify the amount and location of leakage in AMD. “With the advent of OCT-A, we can now visualize the structure of the CNV more clearly, as details are not obscured by dye leakage. We can see if a CNV complex is composed primarily of finer capillary vessels or has a more branched, arteriolized appearance.”
The ability to classify CNV may help determine if certain subtypes may be more responsive to anti-VEGF therapy. For example, Dr. Yiu said, a CNV with mature, large-caliber vessels may be more resistant to anti-VEGF therapy and may be a better candidate for pegpleranib (Fovista), an agent that blocks platelet-derived growth factor and is currently undergoing clinical trials.
EDI-OCT for tumors. Most ocular tumors, malignant or benign, originate in the choroid. In the past, tumors were measured with B-scan ultrasound, but shallow tumors were hard to image. EDI-OCT makes it possible to characterize small tumors in the choroid. The technology also provides a way to identify lesions arising from the sclera, such as sclerochoroidal calcifications, which are benign, Dr. Yiu said.
EDI-OCT for uveitis. The use of EDI-OCT in uveitis is in early stages. For instance, in the case of Vogt-Koyanagi-Harada syndrome, in which marked thickening of the choroid occurs, it is unclear how much of this thickening is due to inflammation or exudation, Dr. Yiu said. Treatment of this disorder may result in thinning of the choroid, although researchers don’t know whether this is a treatment effect or natural progression of disease resolution.
 |
|
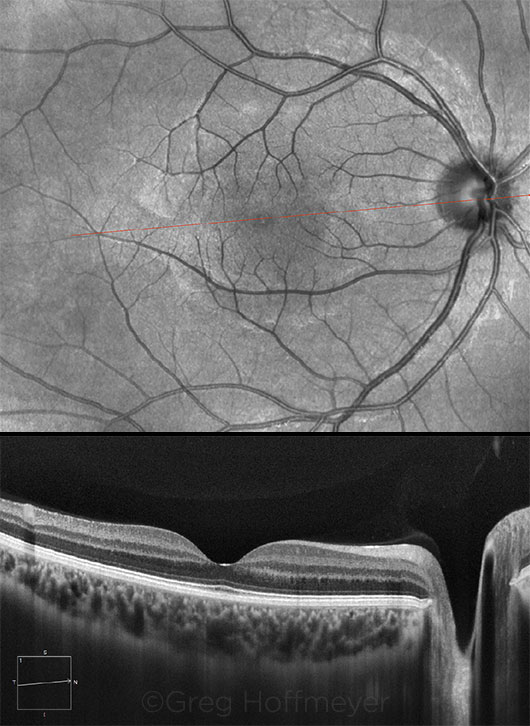
NO PATHOLOGY. Results of a 9-mm EDI-OCT scan over the macula and optic nerve of a 22-year-old healthy female.
|
What’s Next?
Ophthalmologists are at that peculiar juncture where technology outpaces their ability to harness it. As with many technical advances, the answers to the key question—how does this help my patients?—are still evolving, said Mr. Hoffmeyer.
“I would ask clinicians to look, as best they can, at the choroid with the devices they have and to pay attention to what’s happening there,” Dr. Lutty said. Dr. Mullins agreed, adding that it would be “tremendously useful” if more ophthalmologists collected data on the choroid and analyzed that information with regard to treatment outcomes. “We think we’re starting to understand, better than ever before, why patients are going blind. This is a major step along the way to finding and applying new cures.”
___________________________
1 McLeod DS et al. Invest Ophthalmol Vis Sci. 2009;50(10):4982-4991.
___________________________
Mr. Hoffmeyer is senior clinical relations manager at Carl Zeiss Meditec. Relevant financial disclosures: Carl Zeiss Meditec: E.
Dr. Lutty is the G. Edward and G. Britton Durrell Professor of Ophthalmology and director of the Ocular Vasculogenesis and Angiogenesis Laboratory at Wilmer Eye Institute in Baltimore. Relevant financial disclosures: None.
Dr. Mullins is the Martin and Ruth Carver Chair in Ocular Cell Biology and professor of ophthalmology and visual sciences at the University of Iowa, Iowa City. Relevant financial disclosures: None.
Dr. Yiu is assistant professor of ophthalmology at the University of California, Davis. Relevant financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.
Research Update
Some areas under investigation include the following.
Choriocapillaris in AMD. Dr. Mullins is trying to determine which cells in the choriocapillaris are damaged early in the disease process and which survive. Using donor eyes with early AMD and age-matched controls, his lab has found that a larger number of drusen corresponds to a greater loss of vasculature in the choriocapillaris. This suggests a link between blood vessel death and the earliest indication of AMD, he said. “We’re finding more and more evidence that AMD is a disease that afflicts the choroid and strikes it early.”
The complement system. This system of proteins, which is activated when it recognizes a foreign pathogen, can become overactive. Knowing that, Dr. Mullins questioned whether it might be overactive in a person with AMD. The answer: The membrane attack complex was more abundant in patients with AMD. “And the location is right at the choriocapillaris,” he said. “We know the complement system is pushing AMD along.”1
This insight runs contrary to the theory that AMD is an RPE disease, Dr. Mullins said. Yet in terms of the complement system, it appears that the choroid is the site of injury in many patients with AMD. “In these patients, the RPE probably died because the choroid failed first.” Now Dr. Mullins and his colleagues are exploring ways to repair a damaged choroid using choroidal endothelial cells generated by induced pluripotent stem cells, which are taken from any tissue in the patient and genetically modified to behave like embryonic stem cells.
Some day, he said, researchers may find drugs to protect the choroid in early disease from further degeneration caused by an overactive complement system. In more advanced disease, where the loss of the choroid is more substantial, cell replacement therapy will be necessary. These advanced eyes may also have loss of RPE and photoreceptor cells, he added.
Antiangiogenesis. Dr. Lutty’s lab focuses on angiogenic factors that stimulate new blood vessels as well as on naturally occurring inhibitors of angiogenesis. Recent studies demonstrate that 3 antiangiogenic factors (thrombospondin-1, endostatin, and pigment epithelium–derived factor) are reduced or missing, making the AMD choroid susceptible to choroidal neovascularization.2
Dry AMD. Dr. Lutty is working in animal models of dry AMD to see whether it is possible to replace cells lost to the disease, including the cells that make up the choroidal blood vessels. Because no drug can replace these cells (the photoreceptors, RPE, and choriocapillaris), he believes that we must rebuild this complex with several types of progenitors, including those for photoreceptors and RPE cells. “We know that choroidal vasculature is affected in both wet and dry AMD. And there’s a group of retinal degenerations in which the choroid dies,” such as retinitis pigmentosa and Stargardt disease, Dr. Lutty said. “If there’s a drug for dry [AMD], it’s only going to stop the progression of atrophy, but it will still leave an atrophic region where you’ve lost the 3 cell types already,” he added. The future, he said, holds the potential for gene therapy to stop further degeneration and death of surviving photoreceptors.
___________________________
1 Mullins RF et al. Am J Pathol. 2014;184(11):3142-3153.
2 Bhutto IA et al. Arch Ophthalmol. 2008;126(5):670-678.
|