By Lori Baker-Schena, MBA, EdD, Contributing Writer, interviewing Ashley D. Deemer, OD, FAAO, Mark S. Humayun, MD, PhD, Robert W. Massof, PhD, Lotfi B. Merabet, OD, PhD, MPH, Bernhard A. Sabel, PhD, and John D. Shepherd, MD
Download PDF
Recent advances in cameras, displays, and computing power are expanding the options for the approximately 1.8 million Americans living with low vision, a number estimated to increase by an additional 220,000 each year over the next 30 years.1 Moreover, studies on the brain’s role in functional visual impairment, including research on the brain’s neuroplasticity,2 raise the possibility of eventual gains in vision.
The upshot: These advancements “are opening new avenues for patients with low vision,” said Bernhard A. Sabel, PhD, at Otto-von-Guericke University of Magdeburg, Germany. “It is time to be more optimistic about the future—there is more light at the end of the tunnel of low vision and blindness.”
More research needs to be done, and there are significant issues of accessibility, cost, and insurance coverage to consider. But despite these caveats, “The growing popularity of virtual reality (VR) and augmented reality (AR) has the potential to directly benefit patients with low vision, with research focusing on customized strategies involving contrast enhancement, image motion compensation, image remapping, binocular disparity, and eye-tracking capabilities,” said Ashley D. Deemer, OD, FAAO, at the Wilmer Eye Institute in Baltimore.
John D. Shepherd, MD, at the University of Nebraska in Omaha, agreed. These devices “will add to our arsenal for assisting our patients in overcoming the impairment caused by their vision loss. Patients do and will appreciate more options that they can compare with traditional optical and electronic [magnification] devices.”
 |
|
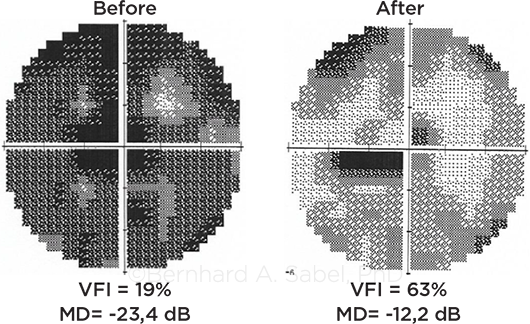
BEYOND DEVICES. Noninvasive brain stimulation aims at activating residual vision and improving visual fields, as shown in this glaucoma patient.
|
Virtual Reality
Novel devices. In VR, the introduction of smartphone technology—which allows for real-time vision processing as long as the patient carries a wearable battery unit—combined with the development of head-mounted VR displays, has led to the development of a number of devices. These include the following:
IrisVision. This device was developed by IrisVision with support from a NEI grant and through collaboration with researchers at Johns Hopkins, Stanford, and UPMC Pittsburgh. It pairs a Samsung smartphone with a goggle-like VR headset and is priced at nearly $3,000.
Borrowing some of the early ideas of image remapping, the device provides customized, variable magnification akin to a virtual bioptic telescope, said Robert W. Massof, PhD, at Johns Hopkins.
The full-field zoom can be adjusted to specific needs such as the loss of central vision, Dr. Deemer said. Other features include a voice-enabled personal assistant that allows the device to become hands free; a function that reads text to the user straight from a document; and a video player for streaming videos connected to Wi-Fi.
eSight 3. Developed by eSight eyewear, this device provides the same functionality as IrisVision and is the only head-mounted magnification system that can be worn while a person is on the go. Current pricing is nearly $6,000.
NuEyes Pro. Developed by NuEyes, this device features smartglasses that are lightweight, wireless, and voice activated and provide a 42-degree field of view. Cost of the most recent version (the Pro 2) is approximately $4,500.
SeeBoost. Designed by SeeBoost for patients with central vision loss, this lightweight electronic screen attaches to prescription eyeglasses. It provides magnification adjustable from 1.4× to 8×, allowing patients to use their peripheral vision, and costs approximately $3,500.
Jordy. This battery-powered headset from Enhanced Vision Systems weighs 8 ounces and features a high-definition autofocus camera for distance, intermediate, and near viewing. Other features include 10× optical zoom and 4× digital zoom, widefield dual viewfinders, and brightness control with five levels. It’s priced at $2,500.
Keys to success. Successful use of these devices often depends on customizing the features to an individual’s unique needs and providing that person with training, Dr. Deemer said. In addition, she said, “We are finding that usage may be affected by device simplicity, especially in older adults.” She is studying usage data to assess the value patients place on system features, functions, and operating parameters.
It’s important to note that some patients may feel awkward being out in public with a head-mounted device, Dr. Deemer cautioned, as they are reluctant to bring attention to themselves and may fear any associated stigma.
And again, cost must be taken into account. As Dr. Shepherd pointed out, “Patients not only are concerned about how they function with the device and how it enables them to participate in a favored activity but also will weigh the benefit they receive relative to the cost of the device.”
An Earlier Prototype
According to Dr. Massof, the late 1980s provided the “perfect storm” for the introduction of the head-mounted video display systems known as Low Vision Enhancement System (LVES) devices.
Researchers had proposed that patients with central blind spots or peripheral vision loss could benefit from image remapping, where image information that would otherwise be lost due to the associated field defect could be distributed onto the still-functioning retina.1 “In the meantime, NASA was developing electronic image remapping technology that could move an image from one system to another, and Johns Hopkins was getting into technology transfer,” said Dr. Massof. “We approached NASA and obtained their help” in developing a LVES prototype.
The first LVES devices consisted of a battery-powered, binocular head-mounted video display equipped with three video cameras and an external video input. The displays were two black-and-white cathode ray tubes mounted in the temple arms of the headset, a reflection of the limited technology at that time, Dr. Massof said.
These devices provided some improvement in activities of daily living, but they did not replace the optical aids available at that point—and they eventually disappeared from the marketplace. Even so, the work on LVES was not without value: “One of the benefits of the LVES project was that it gave low vision a huge amount of attention, and it resulted in increasing awareness of the challenges these patients face,” Dr. Massof said.
___________________________
1 Loshin DS, Juday RD. Optom Vis Sci. 1989;66(6):389-395.
|
Augmented Reality
Whereas VR refers to immersing a low vision patient in a computer-generated environment, AR involves graphic overlays on, or graphic objects inserted in, live renderings of the real, camera-captured environment.3 The goal of this digitized visual space is to enhance patient mobility by helping individuals navigate their environment.
SLAM technology. “AR devices use SLAM (simultaneous localization and mapping) technology,” said Mark S. Humayun, MD, PhD, at the University of Southern California (USC) Ginsburg Institute for Biomedical Therapeutics and Roski Eye Institute in Los Angeles. This involves “computational construction or updating of a map of an unknown environment while keeping track of the person in the location,” he said.
“Think Pokémon Go,” Dr. Humayun added. “The Pokémon do not exist, but by keeping track of a mailbox, for example, the game puts the Pokémon on the mailbox. Autonomous navigation also uses SLAM technology.”
Retinitis pigmentosa research. Patients with retinitis pigmentosa (RP), especially those who have an advanced stage of the disease, have challenges with mobility and may collide with obstacles, especially in low light. They also may have poor dark adaptation and difficultly grasping objects.
Using SLAM, Dr. Humayun and his team at USC created AR-adapted glasses for low vision patients with RP. The glasses fully render the 3-D structure of a room in real time and then generate a semitransparent overlay that highlights potential obstacles with bright colors. This gives patients a better understanding of spatial and depth perception, Dr. Humayun said.
“We took objects that were closer and gave them a white outline, and objects that were further away were outlined in red,” he said. This “starts to give patients that depth information, which is critical.”
The USC researchers conducted a trial of the glasses in 10 patients with RP. The study evaluated patients’ performance in two tests: navigating a functional obstacle course and grasping objects. With the AR glasses, patients averaged 50% fewer collisions on the course and demonstrated a 70% increase in grasp performance.4
“This type of technology does not have to be a bulky headset,” Dr. Humayun said. “And AR can provide a lot of information to patients with visual disabilities if you can overlay content information on the real world.”
Brain Research
In seeking to expand low vision options, researchers are looking beyond the function and mechanics of the eye, exploring the role of the brain and blood vessel dysregulation in vision loss. “The eye and visual system cannot be viewed in isolation but instead need to be studied holistically in the context of the brain and vascular systems,” said Lotfi B. Merabet, OD, PhD, MPH, at Massachusetts Eye and Ear and Harvard in Boston.
Focus on neuroplasticity. Dr. Merabet was inspired to research low vision from his work with visually impaired children. “We know that there are not only ocular causes of visual impairment in children but also neurological causes,” he said. “We just can’t focus on visual acuity and make assumptions based on reading letters on an eye chart. We are working to develop novel, neuroscience-inspired approaches to investigate functional visual deficits using VR assessments based on naturalistic settings.”
Dr. Merabet has studied patients with cerebral visual impairment, with the goal of exploring visual processing deficits and neuroplastic changes in these patients and in those with ocular-based visual impairment.5 “Focusing on neuroplasticity and the compensatory-based behaviors of the brain is a fundamental shift in how we study vision loss,” he said. “We seek to go beyond just the optics of vision.”
Evaluating the eye-brain-vascular triad. Drs. Merabet and Sabel, along with Josef Flammer, MD, have also demonstrated how modulating brain functional networks and improving vascular regulation might lead to the restoration of vision.2
“Most [low vision] patients have some residual vision that is not lost but impaired,” Dr. Sabel said. “Brain degeneration can affect function on the eye-to-brain axis. It becomes more complex with the loss of neurons. However, some of these neurons do not die. But they are not healthy enough to work, so they stay silent.”
He explained that potassium is released when neurons fire action potentials, and this potassium release is sensed by the tiny microcirculation blood vessels, causing them to dilate. This increases blood flow, enhancing glucose and oxygen delivery downstream to support the neurons. However, when the blood vessels do not respond properly because of “vascular dysregulation,” the neurons are low on oxygen and glucose, so they stay silent.
“It is like when you step on the gas in your car but the fuel line is obstructed,” Dr. Sabel said. “The motor can be started with a trickle of fuel, but you cannot drive. Similarly, when a visual stimulus hits the retina in low vision, many ‘silent cells’ are still there.” While these cells are too healthy to die, he said, they are “not healthy enough to fire action potentials because the blood supply is not working properly due to vascular dysregulation. The function is lost, but the neurons are still there.”
As a result, he said, “we have an eye-brain-vascular triad responsible for optimizing residual vision. Our goal is to optimize this residual vision in two ways: by enhancing synaptic transmission by forcing silent neurons to fire neuronal electric signals and—at the same time—by improving blood circulation to wake up these silent neurons.”
Dr. Sabel and his colleagues have investigated whether noninvasive electrical brain stimulation can “awaken” the silent neurons. In a prospective sham-controlled study of partially blind patients with optic neuropathies, they found that 70% of those who received the active treatment noticed improvements in their visual functions, with average improvements of about 24% of the whole visual field and 60% of the damaged area.6 The treatment, offered in Germany, costs $5,000.
Academy Resources
The Academy’s low vision initiative includes patient education materials and Preferred Practice Pattern guidelines. See aao.org/low-vision-and-vision-rehab for more information.
|
Looking Ahead
Dr. Merabet noted that gathering evidence-based approaches to low vision rehabilitation “is a slow process, and it takes a long time to demonstrate efficacy,” he said. “Yet we are making progress in our clinical studies, with the goal of developing strategies to help both children and adults manage their low vision challenges.”
And Dr. Humayun predicted that the field will continue to advance, fueled by a greater understanding of sensory science and neuroscience, along with neuroengineering. From a commercial standpoint, he said, “video games will drive the VR innovations in low vision devices, and autonomous navigation will drive the AR space—all to the benefit of the visually impaired.”
___________________________
1 Chan T et al. JAMA Ophthalmol. 2018;136(1):12-19.
2 Sabel BA et al. Restor Neurol Neurosci. 2018;36(6):767-791.
3 Deemer AD et al. Optom Vis Sci. 2018;95(9):694-703.
4 Angelopoulos AN. Sci Rep. 2019;9:11230.
5 Bennett CR et al. Neurosci Biobehav Rev. 2020;108:171-181.
6 Gall C et al. PLoS One. 2016;11(6):e0156134.
___________________________
Dr. Deemer is assistant professor of ophthalmology at the Wilmer Eye Institute in Baltimore. Relevant financial disclosures: NEI: S.
Dr. Humayun is Cornelius J. Pings Chair in Biomedical Sciences, professor of ophthalmology, biomedical engineering, and integrative anatomical sciences, codirector of the USC Roski Eye Institute, and director of the USC Ginsburg Institute for Biomedical Therapeutics in Los Angeles. Relevant financial disclosures: None.
Dr. Massof is professor of ophthalmology and neuroscience at Johns Hopkins University School of Medicine in Baltimore. Relevant financial disclosures: IrisVision: C; SeeBoost: C.
Dr. Merabet is associate professor of ophthalmology at Harvard and director of the Laboratory for Visual Neuroplasticity at Massachusetts Eye and Ear in Boston. Relevant financial disclosures: Deborah Noonan Memorial Foundation: S; NEI: S; Research to Prevent Blindness: S.
Dr. Sabel is professor of medical psychology at the Institute of Medical Psychology at Otto-von-Guericke University of Magdeburg, Germany. Relevant financial disclosures: Savir: C,O.
Dr. Shepherd is assistant professor of ophthalmology and director of the Weigel Williamson Center for Visual Rehabilitation at the Truhlsen Eye Center, University of Nebraska in Omaha. Relevant financial disclosures: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Deemer NEI: S.
Dr. Humayun 1Co: C,O,P; Alcon: C,L; Allergan: C,L; Clearside: C; Duke Eye Center: P; Eyemedix: C,O,P,S; Iridex: P; Johns Hopkins University: P; Lutronic Vision: C,O; MTTR: C,O; Regenerative Patch Technologies: C,O,P; Replenish: C,O,P; Santen: C,L; Second Sight: O,P; University of Southern California: E,P.
Dr. Massof Iris Vision: C; Janssen: C; SeeBoost: C.
Dr. Merabet Deborah Noonan Memorial Foundation: S; NEI: S; Research to Prevent Blindness: S.
Dr. Sabel Savir: C,O.
Dr. Shepherd None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|