Download PDF
When you think of ophthalmic malpractice claims stemming from misdiagnoses, you might think of rare diseases or unusually challenging complications. But as a recent study conducted by the Ophthalmic Mutual Insurance Company (OMIC) found, that’s far from the case.1,2
OMIC has documented an uptick in legal claims related to diagnostic errors, and this increase is being propelled by what most ophthalmologists would consider a relatively common condition: retinal detachment (RD).
A Surprising Finding
For the OMIC study, Anne M. Menke, RN, PhD, reviewed 1,613 ophthalmic malpractice claims that were either closed or resolved during a 7-year period ending in 2014. It’s fair to say that the results were not what she expected. Of these claims, 223 (nearly 14%) involved allegations of diagnostic error. The biggest surprise? Of this group, 84 (38%) involved the retina, and 65 (29%) specifically involved RDs.2
“When we look at the clinical categories of diagnostic error, retina claims far exceed all other types in both number and percentage,” said Dr. Menke, OMIC patient safety manager, who is based in San Francisco. “And by far, the most frequently missed diagnosis in our entire study was RD—nothing else came close.”
These numbers are concerning, she said. “Most ophthalmologists will think, ‘I already know about retinal tears and detachment. Of course, I know how to make the proper diagnosis.’ But this condition is clearly presenting diagnostic challenges to many ophthalmologists. Why is that?”
 |
|
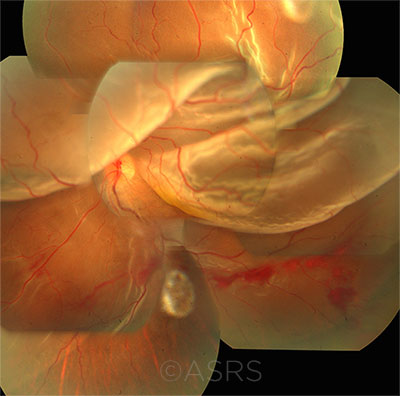
TRAUMA. This patient presented with globe perforation and an RD. Penetration can be seen below the inferior arcade, and some scattered hemorrhaging is evident. This image was originally published in the ASRS Retina Image Bank. Manish Nagpal, MD, FRCS, and Rakesh Juneja. Globe Perforation With Retinal Detachment. Retina Image Bank. 2017; Image Number 26957. © The American Society of Retina Specialists.
|
Slipping Through the Cracks
An early diagnosis of an RD is key, as the rate of successful reattachment is higher—and the visual results are better—when repair comes early, especially before the detachment involves the macula.
But as Dr. Menke pointed out, 85% of the RD patients in the OMIC study who were misdiagnosed did indeed present with risk factors specific to RD (see “Who’s at Risk?”). How could so many ophthalmologists fail to diagnose this subset of RD patients? As with many malpractice issues, the misdiagnosis of an RD is often much more than an issue of clinical acumen; other factors can trigger a cascade of errors.
Need for a well-run team. The proper diagnosis of an RD takes the coordination of a well-educated and engaged team, said Ann A. Warn, MD, MBA, a comprehensive ophthalmologist in Oklahoma City, and the first thing that team needs to do is to obtain an adequate history and recognize the risk factors.
“We as ophthalmologists may know everything there is to know about RDs,” said Dr. Warn, “but there’s always that chance of things falling through the cracks on a busy day. That’s why it’s so important to have multiple levels of teamwork where nonmedical staff act as the gateway to make the first decisions, catch the risks, and bring [the case] to the attention of the ophthalmologist.”
Staff etiquette is as important as staff education, added Dr. Menke. “Yes, your team must be informed—they need written protocols to channel patients to the ophthalmologist on time and they need to know the importance of a change in flashes or floaters. But politeness is also paramount. The phones might be ringing off the hook and the front desk [staff] may be in a rush to leave for their kid’s soccer game. But the team’s first job is to provide kind care to everyone. Unwelcoming or brusque staff can quickly push patients away or prevent them from making the necessary follow- up visits.”
Need for patient education. Well-informed patients can help you and your staff tease out the correct diagnosis and ensure that they keep track of their symptoms and return for any necessary follow-up exams. “Simply put,” said Dr. Warn, “you can’t get the information you need from patients if they don’t understand the risk factors or [know] what symptoms they should be looking for.”
The experts recommended providing patients with clear instructions for monitoring and reporting worrisome changes in vision and using language they can understand. “If you ask a patient on the phone, ‘Are you a high myope?’ they may not have a clue what you are talking about,” said Dr. Warn. “They also may not understand what you mean by ‘family history’ until you ask directly about the health of their mother or father.
Dr. Warn emphasized, “These patients aren’t physicians and aren’t perfect historians—they may not even recall eye trauma from 5 years ago. But it’s up to us to help them communicate so we can draw out what we need to make the proper diagnosis.”
Need for a focused physician. The physician’s decision-making process and focus are also key. “Ten out of 10 well-trained ophthalmologists know the risk factors for RD, so this is not a question of a knowledge gap,” Dr. Menke said. “But what’s the interference when they’re with the patient? That’s the real issue.”
 |
|
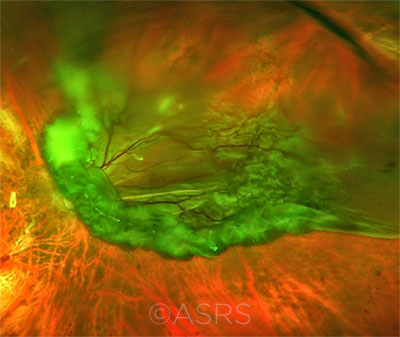
AWARENESS. Even though most PVDs don’t develop into a full tear (seen here), the experts warn against letting down your guard. This image was originally published in the ASRS Retina Image Bank. Jason S. Calhoun. Full Retinal Detachment. Retina Image Bank. 2015; Image Number 25723. © The American Society of Retina Specialists.
|
A Critical Factor: The Attention Gap
Dr. Menke admitted that although many factors are likely to have an impact on patient encounters, the most significant might be the competition for the ophthalmologist’s attention. In other words, she asked, is the physician distracted during the diagnostic process?
The practice of medicine is in flux and is increasingly complicated by outside variables. Ophthalmologists are forced to comply with a growing number of ever-changing rules and regulations. At the same time, more and more ophthalmologists are taking patient calls and texts on their smartphones at the clinic. Add in staff interruptions, and the distractions multiply.
“During the diagnostic process, your brain has to be able to retain the information you’re taking in,” said Dr. Menke. “There’s simply not enough memory space for a physician to stay focused on a patient’s complaint and history when attention keeps being diverted away from them.”
What’s the fix? “It’s really a question of how to be Zen and live in the moment,” she said. “By staying present during each and every patient encounter, you’re better able to stay focused on important aspects of early RD diagnosis: obtaining a thorough history, conducting a proper examination, and understanding when you should refer to a specialist.”
 |
|
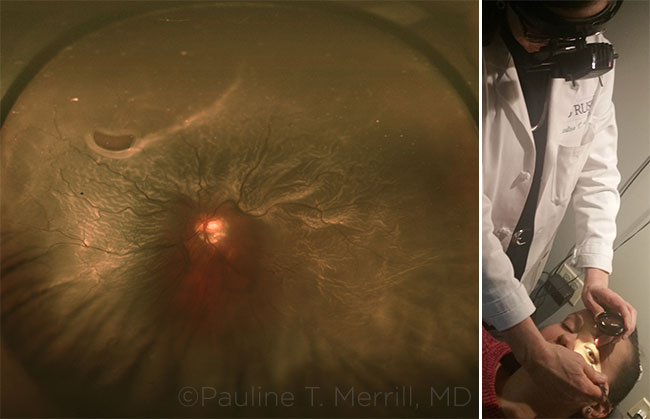
FINDINGS. This Optos photo (left) is of a large RD from a relatively small retinal tear and shows the characteristic corrugated appearance of the detached retina. Scleral depression (right) is essential for at-risk patients.
|
Who’s at Risk?
The risk factors for developing RD and the importance of periodic follow-up are outlined in the Academy’s Preferred Practice Pattern on the topic.3 At-risk patients who experience new changes in vision—such as a decrease in visual acuity, loss of visual field, or increase in floaters—should notify their ophthalmologist promptly.
Posterior vitreous detachment. The primary pathogenic mechanism—and the biggest risk factor—for RD is PVD. Any patient who presents with a PVD should be considered at risk for a retinal break or tear and, therefore, an RD.
Myopia. More than half of RDs occur in myopic eyes, and the risk increases as the axial length increases. Even low myopes (1 to 3 D) have an increased risk compared with nonmyopes.
Lattice degeneration. Lattice degeneration, a developmental thinning of the retina, occurs in 6% to 8% of the population. Some 30% of patients with an RD will also have lattice degeneration.
Trauma. Blunt or penetrating injuries to the eye can damage the vitreous and the retina and can therefore increase the risk of RD. The resulting changes in the vitreoretinal interface can present immediately after injury or years later.
In a 2017 study, Brodowska et al. validated the use of the Retinal Detachment after Open Globe Injury (RD-OGI) Score to predict a patient’s future risk of developing an RD.4 For instance, at 1 year, those patients deemed to be in the low-risk RD-OGI group had a 3% RD rate in the derivation cohort and a 0% RD rate in the validation cohort. In contrast, patients in the high-risk RD-OGI group had a 73% RD rate in the derivation cohort and an 86% RD rate in the validation cohort.
Cataract surgery. RDs occur in about 1% of patients following cataract surgery. This increased risk is associated with young age, male sex, long axial lengths, and the occurrence of any surgical complications.
Detachment in the fellow eye. Vitreoretinal changes are oftentimes bilateral. If a patient has a history of nontraumatic detachment in one eye, he or she is at a 10% increased risk of developing an RD in the fellow eye.
Genetic factors. Children born with certain syndromes are genetically predisposed for RD. The most common is Stickler syndrome, a systemic connective tissue disorder resulting in defective collagen production.
Failure to Diagnose RDs
In analyzing the claims related to RDs, the following factors emerged in the OMIC study. The number of those related to the ophthalmologist outweighed staff or other system factors by nearly 2:1.
Ophthalmologist Factors
- Missing documentation—no documentation on dilated exam, positive findings, and/or RD warnings
- Judgment deficiencies—when surgery is needed; when a dilated exam is needed; when to refer; when more work-up is needed
- Diagnostic process deficiencies—what caused vision loss; how to restart process when initial diagnosis is ruled out; no scleral depression performed
- Exam skill deficiencies—did not recognize tear or RD; misinterpreted fundus photo
- Knowledge deficiencies—inadequate knowledge of RD risk factors and natural history, visual fields and RDs, and/or trauma and RD
Systemic Factors
- Poor telephone care—MD not involved; staff given too much authority; call not documented
- No electronic health records carry-forward policy—when to use; when not to use; risk of fraud determination
- Delayed authorization—test and/or referral
- Poor communication with patients—inadequate RD warnings; poor instructions (e.g., regarding travel)
- Credentialing problems—no written protocols for role of employed optometrist (when MD consult or referral needed); complaints from patients, staff, and other MDs not acted upon
Adapted from Menke AM. The OMIC Digest. 2017;27(1):1-5, vi.
|
The Standard of Care
In addition to timely clinical suspicion, the detection of an RD or any retinal pathology that may subsequently lead to an RD requires the correct ophthalmic examination.
“If you’re going to be involved in taking care of patients who are at risk for RD—and that’s virtually every ophthalmologist—you need to know the Academy’s PPP,” said George A. Williams, MD, a vitreoretinal specialist in southeast Michigan. “As it states, the standard of care for any at-risk patient requires a dilated examination of the entire fundus with indirect ophthalmoscopy and scleral depression—period, end of discussion.”
In addition, the exam should include confrontation visual field testing, assessing for the presence of a relative afferent pupillary defect, and inspecting the vitreous for hemorrhage, detachment, and pigmented cells, said Dr. Williams, who is also chair of the OMIC Board of Directors and president-elect of the Academy.
When Referral Is Warranted
“Most comprehensive ophthalmologists will be comfortable with this standard of care,” said Pauline T. Merrill, MD, a vitreoretinal specialist in Chicago. “But there are very important reasons for referring an at-risk patient to a specialist.”
Scleral depression. If you’re unwilling to perform a scleral depression, or if your patient isn’t tolerating the procedure, you should contact a retina specialist to take over. “Some general ophthalmologists haven’t depressed a patient in a long time and just aren’t comfortable doing so,” said Dr. Merrill. “But a proper scleral depression is a necessity for at-risk patients—and that involves clearly visualizing the full extent of the retina all the way out to the ora serrata to identify any tears. If you aren’t accomplishing that, you aren’t performing a complete exam.”
Vitreous hemorrhage. Many patients with retinal tears will present with blood and pigmented cells in the anterior vitreous. If their vitreous hemorrhage obscures all retinal details, the comprehensive ophthalmologist should consider early referral to a specialist who can perform a B-scan evaluation. “If there’s an acute PVD and a vitreous hemorrhage, the risk of retinal tear and detachment increases substantially,” said Dr. Merrill. “If there’s enough hemorrhage that you can’t get a clear view even with scleral depression, refer to someone who can perform an ultrasound and who can follow that patient closely.”
Rule of thumb. Ultimately, said Dr. Merrill, if you’re considering a referral, the general rule is to ask yourself, “Am I comfortable with my examination of the patient and confident that there’s no tear or detachment?” If the answer is “no” for any reason, make the call.
“Comprehensive ophthalmologists should have a low threshold for referral,” added Dr. Warn. “If I can’t get a thorough exam for whatever reason—maybe there’s a bit of hemorrhage, the media is opaque, or I expect a detachment but the diagnostic exam doesn’t match—I’ll refer, especially if there’s any question as to what I’m seeing.”
Need for Vigilance
During a normal day, the average ophthalmologist might see 2 or 3 PVDs, most of which don’t involve a retinal tear and won’t develop into an RD. “But don’t get lulled to sleep by thinking ‘It’s just a PVD,’” said Dr. Williams. “If the patient presents with [classic] warning signs, you need to take that extra-careful look, even if you think you probably aren’t going to find anything.”
And know the Academy’s PPP recommendations backward and forward, Dr. Menke recommended. Although the PPP introduction states that the guidelines “do not establish the legal standard of care,” Dr. Menke pointed out that “the lawyers for patients will have read everything. If you are sued, the plaintiff’s attorney will ask you about relevant clinical guidelines and will want to know why you didn’t follow the PPP. Understanding and implementing these recommendations will protect your patient and may keep you out of court.”
___________________________
1 Menke AM. The OMIC Digest. 2016;26(2):1-5, vi.
2 Menke AM. The OMIC Digest. 2017;27(1):1-5, vi.
3 American Academy of Ophthalmology Retina/Vitreous Panel. Preferred Practice Pattern. Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration. San Francisco, Calif.: American Academy of Ophthalmology; 2014. Available at aao.org/ppp.
4 Brodowska K et al. Ophthalmology. 2017;124(5):674-678.
Research Update: A Role for Artificial Intelligence?
Recent studies have found that computer-based image analysis is highly accurate in detecting retinal disease.1,2 Before long, artificial intelligence (AI) may offer diagnostic assistance for RDs as well.
The promise. AI in the field of health care is being spearheaded by Google and IBM as well as academic institutions and startups. And the reason for their focus on ophthalmology is simple: The specialty offers advanced imaging methods that lend themselves to advanced imaging analytics.
“With these companies’ algorithms in tow, an AI machine in the cloud can scan an image, locate the biomarkers of disease, analyze these biomarkers, and help the ophthalmologist determine what they are seeing in terms of pathology,” said oculoplastics surgeon P. Lloyd Hildebrand, MD, FACS, who is based in New York City and consults with the IBM Watson Health AI project. “And although this type of machine learning is more commonly associated with diabetic retinopathy and macular degeneration, AI assistance with RD is on the horizon.”
This potential was recently demonstrated by Japanese researchers.3 Using ultra-widefield fundus ophthalmoscopy, they found that their deep learning algorithm demonstrated a high sensitivity and high specificity for the early diagnosis of RDs in 411 images from 407 patients.
The limitations. Although these findings are significant, they also cast light upon some of AI’s current shortcomings. “The value of AI is largely dependent on the quantity and quality of available images,” said Ehsan Rahimy, MD, a vitreoretinal specialist who practices in Palo Alto, California, and consults with the Google Brain AI team. “To help the AI learn and adapt, you want to feed it with lots and lots of images.”
Not enough RD images? These large image libraries currently exist for diseases like diabetic retinopathy and macular degeneration, because their detection involves standard fundus photography. RDs are different, though, noted Dr. Rahimy. “The ultra-widefield cameras used by the Japanese team are relatively new, so it takes time to build up the same robust datasets.”
Not enough good images? Image quality is another concern. “If a patient has a severe vitreous hemorrhage or a dense cataract, the media may be unclear, and it becomes a challenge to capture a suitable image for the AI,” said Dr. Rahimy. “Ultra-widefield cameras can also result in the creation of false artifacts that may mimic peripheral retinal pathology such as a tear or RD. But these problems are ultimately fixable over time as we train the algorithms how to interpret and decipher anomalies from real pathology.”
___________________________
1 Gulshan V et al. JAMA. 2016;316(22):2402-2410.
2 Ting DSW et al. JAMA. 2017;318(22):2211-2223.
3 Ohsugi H et al. Sci Rep. 2017;7(1):9425.
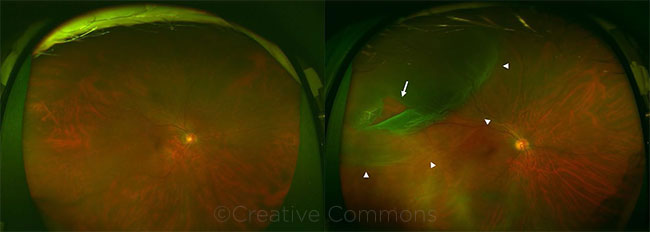
COMPARISON. These ultra-widefield images are of eyes without (left) and with (right) an RD. The white arrow indicates the retinal break, and the arrowheads indicate the areas of detachment. Hideharu Ohsugi, Hitoshi Tabuchi, Hiroki Enno, and Naofumi Ishitobi. Sci Rep. 2017;7(1):9425. Reprinted under Creative Commons License Attribution 4.0 International License. Caption slightly adapted from original.
|
Meet the Experts
P. Lloyd Hildebrand, MD, FACS In practice with Union Square Eye Care in New York, N.Y., and consultant to the IBM Watson Health AI project. Relevant financial disclosures: IBM: C.
Anne M. Menke, RN, PhD Patient safety manager at OMIC in San Francisco. Relevant financial disclosures: OMIC: E.
Pauline T. Merrill, MD Assistant professor of ophthalmology at Rush University Medical Center and partner at Illinois Retina Associates in Chicago. Relevant financial disclosures: None.
Ehsan Rahimy, MD Surgical and medical vitreoretinal specialist at the Palo Alto Medical Foundation in Palo Alto, Calif., and consultant to the Google Brain AI team. Relevant financial disclosures: Google: C.
Ann A. Warn, MD, MBA Associate professor of ophthalmology at the Dean McGee Eye Institute in Oklahoma City. Relevant financial disclosures: OMIC: C.
George A. Williams, MD Vitreoretinal specialist in southeast Michigan and chair of the OMIC Board of Directors. Relevant financial disclosures: OMIC: C.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
P. Lloyd Hildebrand, MD, FACS IBM: C.
Anne M. Menke, RN, PhD OMIC: E.
Pauline T. Merrill, MD AbbVie: C,S; Alimera Sciences: C,S; Allergan: C; Covalent: O; EyeGate Pharma: S; National Eye Institute: S; OMIC: C; pSivida: C; Santen: C,S.
Ehsan Rahimy, MD Allergan: C; Google: C.
Ann A. Warn, MD, MBA American Board of Ophthalmology: C; OMIC: C.
George A. Williams, MD OMIC: C.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|