By Joel Kovoor, BS, Joseph F. Panarelli, MD, and Anna T. Do, MD
Edited By: Bennie H. Jeng, MD
Download PDF
Ocular hypotony is an uncommon but potentially vision-threatening event defined as either an intraocular pressure (IOP) that is 3 standard deviations below normal (<6.5 mm Hg) or an IOP low enough to cause visual impairment. This condition often manifests in the form of hypotony maculopathy (Fig. 1), corneal edema, astigmatism, choroidal effusion, and/or accelerated cataract formation. Treatment is typically reserved for patients with visual impairment and is often not required for those with asymptomatic “statistical” hypotony.
Etiology. Hypotony has numerous possible etiologies, which can be broadly classified in two groups: decreased aqueous production or increased aqueous outflow. Hypotony related to decreased aqueous production can be caused by ischemia of the ciliary body, uveitis, and tractional ciliary body detachment. Hypotony related to increased outflow can result from cyclodialysis clefts, retinal detachment, and iatrogenic causes such as glaucoma filtering surgeries, among others. Thus, management is specific to the etiology and relies on a detailed ocular history and exam.
Glaucoma surgery. Hypotony associated with glaucoma surgery is the most common etiology. The reported incidence of clinical hypotony ranges from 1% to 31% following filtering glaucoma surgery.1,2 The most frequent causes of postsurgical hypotony are, in order, bleb leak, post-trabeculectomy overfiltration, use of glaucoma drainage devices, and iatrogenic cyclodialysis cleft formation.
 |
|
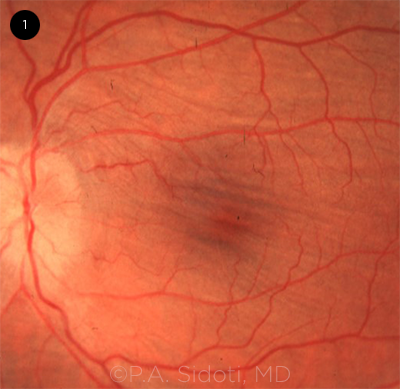
HYPOTONY MACULOPATHY. Chorioretinal macular folds characteristic of hypotony maculopathy are seen on this fundus photograph and can be detected on vertical optical coherence tomography scans.
|
Bleb Leak
Pathophysiology and background. Bleb leaks are primarily caused by poor conjunctival integrity. Early bleb leaks (days to weeks after trabeculectomy) are often the result of inadvertent buttonhole tears or conjunctival retraction, whereas late bleb leaks (months to years after trabeculectomy) most commonly occur when focal, thin, avascular blebs erode or rupture. Antifibrotics such as mitomycin C and 5-fluorouracil are well-documented risk factors for late bleb leaks and must be used judiciously. Diagnosis of bleb leakage is typically confirmed with the Seidel test.
Management: early leak. The management approach to an early bleb leak depends largely on the extent of leakage. In cases of hypotony with a small amount of leakage, deep anterior chamber, and minimal change in visual acuity, various conservative measures can be used. These include steroid taper to allow for healing, aqueous humor suppressants to slow the rate of leakage, and/or soft bandage contacts with antibiotics to tamponade the leak and prevent infection. If these efforts fail, autologous blood injection and cyanoacrylate or fibrin glue have been shown to help, but outcomes are variable.
In the more urgent cases—those with shallow anterior chambers and worse visual symptoms—resuturing of the wound with or without resuturing of the underlying scleral flap should be considered. Using compression sutures to wall off the leaking area is an alternative; however, adding sutures may lead to scarring and limit the bleb flow in the future.
Management: late leak. Management of late leaks depends on the severity of the condition; the conservative measures described for early leaks may be tried when appropriate. However, late leaks often require surgical intervention such as conjunctival advancement. One method involves advancement of healthy conjunctiva over a denuded bleb after stripping the overlying epithelium. In another method, the bleb is entirely excised before conjunctival advancement, but this technique is associated with a higher rate of bleb failure and a greater need for glaucoma medications.1
If there is not enough viable conjunctiva surrounding the bleb for advancement, a free autologous graft from another site in the same eye or from the fellow eye can be used. Success in treating bleb leak or hypotony with conjunctival grafts has been reportedto be 75.8%.2 As an alternative, amniotic membrane can be used, but it may have slightly lower rates of long-term graft survival compared with standard conjunctival advancement.3
Overfiltering Bleb
Pathophysiology and background. An overfiltering bleb allows too much aqueous humor to pass through the bleb. Overfiltration can be caused by a loose scleral flap or insufficient inflammatory response at the surgical site, both of which reduce outflow resistance. Patients with overfiltering blebs typically present several years after glaucoma surgery and may be completely asymptomatic or have decreased vision or other signs of hypotony. On examination, the bleb will appear enlarged, elevated, and extended tangentially as well as posteriorly, with no evidence of leakage on Seidel test—even with gentle digital pressure.
Management. As with early bleb leaks, management typically starts with conservative methods, including steroid taper, aqueous humor suppressants, and autologous blood injection. In cases of overfiltration with shallow anterior chambers, cycloplegics (e.g., atropine or cyclopentolate) can be used to deepen the anterior chamber. Medical treatment with a cycloplegic agent will shift the lens-iris diaphragm posteriorly, which will help restart or increase ciliary body aqueous production. These medications can help resolve hypotony in mild to moderate cases of overfiltration, but patients should be warned that their vision may be affected by limited accommodation.
In chronic cases of overfiltration refractory to conservative treatment, surgical techniques such as conjunctival compression sutures can be employed. Compared with more invasive techniques such as direct transconjunctival scleral flap resuturing, compression sutures can easily be removed at the slit lamp to titrate the flow and have the advantage of not piercing the scleral flap itself. According to one retrospective study, conjunctival compression sutures had a 64.4% success rate for total resolution of hypotony at six months.4 Conjunctival advancement and suturing with Vicryl sutures with or without scleral cauterization can also be used to induce additional scarring and reduce overfiltration.
Scleral flap resuturing is another technique, and it can be performed through an open or a transconjunctival approach. In the more traditional open approach, the overlying conjunctiva is reopened and the exposed scleral flap is resutured directly.
In the transconjunctival approach, sutures are passed radially through intact conjunctiva, the scleral flap, and then the adjacent sclera and knotted tightly over the conjunctiva. This technique has the advantage of not reopening the conjunctiva.
If first-line surgical management fails, bleb excision with scleral or pericardial graft can be used with conjunctival advancement.
Glaucoma Drainage Implants
Pathophysiology and background. Glaucoma drainage implants reduce IOP by shunting aqueous humor from the anterior chamber to an equatorial endplate placed in the subconjunctival space. These devices are typically indicated for patients in whom trabeculectomy was unsuccessful or those at high risk of trabeculectomy failure.
Two of the most commonly used drainage implant variants are the Ahmed Glaucoma Valve (AGV) and the Baerveldt Glaucoma Implant (BGI), which are primarily differentiated by the presence of a flow-restricting valve in the AGV. Valveless devices such as BGI are more liable to cause hypotony and require a temporary tube ligature to control IOP until the endplate is encapsulated. Although the incidence of persistent hypotony is greater for the BGI than for the AGV, the absolute rate of hypotony is fairly low: approximately 0.4% and 4.5% for the AGV and BGI, respectively.5 The most common causes of hypotony with glaucoma drainage implants are insufficient endplate encapsulation and reduced production of aqueous humor secondary to postoperative inflammation.
Management. Conservative treatment starts with watchful waiting in cases of transient hypotony without evidence of excessive anterior chamber shallowing or symptomatic vision loss. IOP will often rise as the endplate naturally encapsulates or as postoperative inflammation resolves. Intracameral injection of a cohesive viscoelastic may also be used to help stabilize the eye and allow more time for encapsulation. If hypotony persists, the tube can be ligated with absorbable or nonabsorbable suture to halt outflow. Nonabsorbable sutures can be released in the office by laser transconjunctival ligature lysis as needed for IOP control.
Intraluminal stenting, which involves running a suture segment through the tube lumen, can also be employed to increase resistance by reducing the total cross-sectional area of the tube. This technique can be performed through an ab interno or ab externo approach and has the advantage of easily being removed if IOP becomes too high.6
Ultimately, if hypotony after glaucoma surgery persists, the drainage implant can be removed.
Cyclodialysis Cleft
Pathophysiology and background. A cyclodialysis cleft occurs when the ciliary body is detached from the scleral spur, creating an abnormal pathway between the anterior chamber and suprachoroidal space. Although a cyclodialysis cleft is usually the result of blunt trauma to the eye, it can also be caused iatrogenically during any intraocular surgery that involves manipulation of the anterior chamber and angle structures. Minimally invasive angle procedures such as goniotomy with the Kahook Dual Blade (New World Medical) or Trabectome (MST), gonioscopy-assisted transluminal trabeculotomy, and insertion of an iStent (Glaukos) or Hydrus Microstent (Ivantis) are at higher risk of cyclodialysis cleft than other surgeries.
Many clefts resolve spontaneously, but those that persist require urgent attention to prevent development of further visual deficits. Clefts can be visualized on gonioscopy, ultrasound biomicroscopy, and anterior segment optical coherence tomography.
Management. Cycloplegics can be used as a first step in conservative treatment of small cyclodialysis clefts. These medications work by relaxing the ciliary body, allowing the detached muscle fibers to realign with the scleral spur.
If this does not resolve the cleft, treatment can be escalated to argon photocoagulation, transscleral cyclophotocoagulation, or cryotherapy to promote inflammation at the detachment site, thereby inducing adhesion to seal the cleft. This treatment can be repeated multiple times and has shown good success for smaller clefts (less than 3 clock-hours).7
Direct cyclopexy can be employed for large clefts (3 to 6 clock-hours) or those that failed to close with the preceding measures. Although many techniques have been described for cyclopexy, the most common method involves creating a full-thickness scleral flap to access the ciliary body and using mattress sutures to attach it directly to the undersurface of the sclera. Other techniques include employing a partial or shelved limbal-scleral flap. Long-term results are promising, with one study reporting 100% resolution of IOP for 12 patients who underwent direct cyclopexy with a double lamellar scleral flap.8
 |
|
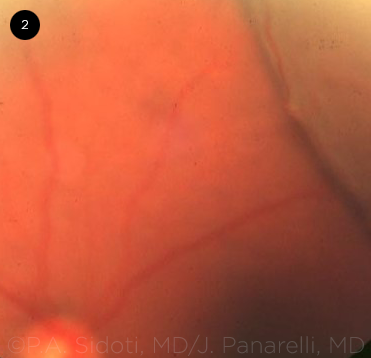
CHOROIDAL EFFUSIONS. Fundus photograph of peripheral choroidal effusion secondary to hypotony following a trabeculectomy with mitomycin C.
|
Related Complication: Choroidal Effusions
Pathophysiology and background. Choroidal effusions are a common and clinically relevant complication of hypotony after glaucoma surgery (Fig. 2). They can present in serous or hemorrhagic form. In serous effusions, low IOP reduces the hydrostatic pressure opposing choroidal vessels, allowing transudative fluid accumulation, whereas rupture of those same vessels will result in a hemorrhagic effusion. Because it may be difficult to differentiate between the two on clinical examination, an ultrasound B-scan should be performed (Fig. 3). Small peripheral effusions are usually asymptomatic, whereas large effusions may extend posteriorly into the macula or may displace the lens-iris diaphragm, leading to decreased vision.
Management. Conservative treatment starts with medical management targeted toward the specific etiology of hypotony. This approach includes treatments such as cycloplegic drops, steroid taper, or bandage contact lenses.
However, for cases in which persistent or large effusions are accompanied by symptomatic vision loss or a flat anterior chamber, surgical drainage is indicated. Choroidal effusion drainage is approached by quadrant and can be repeated if recurrent.
 |
|
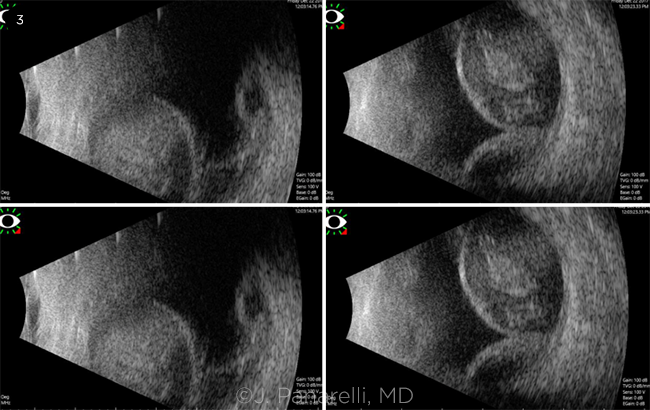
HEMORRHAGIC EFFUSION. B-scan imaging demonstrating large hemorrhagic effusions with low to medium internal reflectivity characteristic of a partially liquefied clot.
|
Conclusion
Glaucoma surgical procedures are the most common cause of ocular hypotony, which can occur as a result of bleb leak or overfiltration, use of glaucoma drainage implants, or iatrogenic cyclodialysis cleft. This rare complication may be asymptomatic or lead to visual loss. Asymptomatic patients with adequate anterior chamber depth may be observed. Hypotony that causes a shallow anterior chamber or visual symptoms is managed according to the etiology and severity, with treatments ranging from medical therapy with aqueous suppressants or cycloplegics to surgical interventions including conjunctival advancement, compression sutures, tube ligation, and flap suturing as appropriate.
__________________________
1 Melo AB et al. J Ophthalmic Vis Res. 2012;7(4):281-288.
2 Panday M et al. J Glaucoma. 2011;20(6):392-397.
3 Budenz DL et al. Am J Ophthalmol. 2000;130(5):580-588.
4 Quaranta L et al. Eur J Ophthalmol. 2013;23(4):593-596.
5 Christakis PG et al; ABC-AVB Study Groups. Am J Ophthalmol. 2017;176:118-126.
6 Feinstein M et al. J Glaucoma. 2018;27(3):e61- e63.
7 Wang Q et al. Surv Ophthalmol. 2019;64(5):619-638
8 Agrawal P, Shah P. Eye (Lond). 2013;27(12):1347-1352.
__________________________
Mr. Kovoor is a fourth-year medical student at the NYU Grossman School of Medicine in New York, N.Y. Dr. Panarelli is director of the glaucoma fellowship and chief of the glaucoma service, and Dr. Do is a clinical assistant professor; both are in the department of ophthalmology at the NYU Grossman School of Medicine in New York, N.Y. Financial disclosures: Dr. Panarelli: AbbVie: C; Aerie: C; CorneaGen: C; Glaukos: C; New World Medical: C; Santen: C. Mr. Kovoor and Dr. Do: None.
See the disclosure key at www.aao.org/eyenet/disclosures.