Download PDF
Steroids are commonly prescribed for various autoimmune and inflammatory conditions and are routinely used after intraocular surgery. Despite numerous benefits, steroids can have adverse systemic and ocular side effects, including cataracts, elevated IOP, and glaucoma. One-third of patients may experience a type of ocular hypertension known as steroid response.
Steroid-induced glaucoma is defined as elevated IOP and glaucomatous optic neuropathy in the setting of corticosteroid use. This iatrogenic disease is often difficult to manage, as patients may require continued steroid treatment for their underlying conditions. Steroid-induced IOP elevation is dependent on the route of administration, potency, dose, treatment duration, and type of steroid, in addition to patient-related risk factors.
Prompt diagnosis and early intervention are critical to prevent glaucomatous optic neuropathy and vision loss. Therefore, physicians must be mindful of the association between steroids and secondary glaucoma.
Epidemiology
The risk of steroid response varies among individuals, which contributes to the unpredictable nature of steroid-induced glaucoma. In the general population exposed to topical ocular steroids, 5% to 6% are high steroid responders (IOP elevation >15 mm Hg), 29% to 36% are moderate responders (IOP elevation between 6 mm Hg and 15 mm Hg), and 58% to 66% do not experience significant IOP elevation.1,2
In addition, Becker and Mills found that patients with primary open-angle glaucoma (POAG) exhibit marked IOP elevation and decreased aqueous outflow facility when exposed to topical ocular steroids.1 Individuals with a first-degree relative who has POAG or with a diagnosis of glaucoma suspect are also more likely to develop ocular hypertension when treated with steroids. Other risk factors include previous steroid response, high myopia, angle-recession glaucoma, very young (<6 years old) or older age, type 1 diabetes mellitus, and connective tissue disease.2
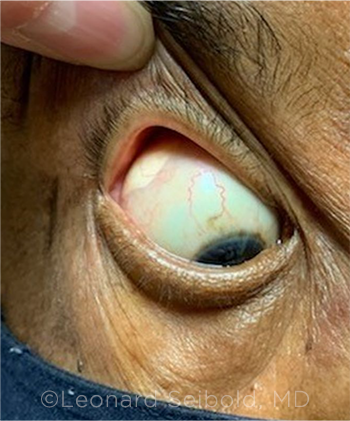 |
|
PERIOCULAR STEROIDS. Sub-Tenon steroid depot in a patient with uveitis resulting in secondary glaucoma.
|
Pathogenesis
Trabecular meshwork morphological and biochemical changes. Steroid-induced glaucoma is considered a secondary open-angle glaucoma. Although the exact mechanism is unknown, the main cause of the disease is increased aqueous outflow resistance at the level of the trabecular meshwork (TM), leading to IOP elevation.2 Accumulation of extracellular matrix proteins including fibronectin, elastin, and hydrated polymerized glycosaminoglycans, as well as mechanical obstruction by steroid particles, is thought to reduce aqueous outflow.
Moreover, steroids inhibit proteases and suppress the phagocytic activity of the TM, thus promoting the accumulation of aqueous debris and obstruction. Steroids also decrease the synthesis of prostaglandins and have a significant effect on the cytoskeleton structure of the TM through formation of cross-linked actin networks.
Genetics. Several genes are associated with steroid-induced glaucoma, including those encoding for myocilin, alpha 1-antichymotrypsin, pigment epithelial–derived factor, cornea-derived transcript factor 6, and prostaglandin D2 synthase.2 Although still poorly understood, myocilin has been extensively studied because of its link to juvenile and adult-onset open-angle glaucoma. Myocilin is highly expressed in trabecular cells exposed to glucocorticoids, and its expression shows a similar dose response and delay in onset as steroid-induced ocular hypertension. Further research into the genetics associated with steroid-induced glaucoma would be beneficial to identify patients at risk.
Steroid Administration
Steroid-induced ocular hypertension and glaucoma can occur after topical, periocular, intraocular, inhaled, nasal, systemic, or transcutaneous administration. Rarely, excess endogenous steroid production can cause ocular hypertension. Steroid delivery and potency are major factors in IOP elevation. In general, topical, periocular, and intravitreal administration account for most cases of steroid-induced glaucoma, with the topical route being the most frequently involved.
Topical steroids. The effect of topical ocular steroids on IOP depends on the potency of the drug formulation. Dexamethasone and prednisolone are more potent steroids. In one study, 0.1% dexamethasone was associated with an average increase of 22 mm Hg from baseline IOP, while prednisolone acetate, a commonly used postoperative medication, demonstrated an average IOP elevation of 10 mm Hg in patients who were previously identified as steroid responders.3 Difluprednate is one of the most potent topical steroids, and approximately 3% of patients using difluprednate experienced an IOP increase of 10 mm Hg or more above baseline, compared with 1% in the placebo group.4
Less potent topical ocular steroids include medrysone 1.0%, tetrahydrotriamcinolone 0.25%, hydrocortisone 0.5%, and fluorometholone 0.1%. On average, these drugs raise the IOP by 1.0, 1.8, 3.2, and 6.1 mm Hg, respectively.3 Newer medications such as loteprednol etabonate and rimexolone have less of an effect on IOP. It is important to note that the incidence rates of steroid-induced ocular hypertension and glaucoma vary among studies because of patient-related risk factors and different definitions of IOP elevation.
Periocular and intravitreal steroids. Periocular and intravitreal steroids include triamcinolone acetonide (TA), fluocinolone acetonide (FA), and dexamethasone (DEX). These medications have a longer duration of action compared with topical steroids. All periocular steroids can increase IOP, and the risk appears to be intermediate between topical and intravitreal administration.
There is limited literature comparing outcomes of different types of periocular steroid delivery, including subconjunctival, sub-Tenon, and retrobulbar injection. Among the periocular steroid routes of administration, sub-Tenon has the highest risk of IOP elevation.
Intravitreal steroids have become more widely used recently because of broader indications and successful outcomes in various conditions. The most popular intravitreal steroids are DEX and TA, with intravitreal TA being the more commonly used. Intravitreal TA has been shown to cause ocular hypertension in 30% to 45% of patients for up to nine months.5 Intravitreal DEX is considered to have a lower risk of steroid response (11%-17%), with a shorter duration (lasting about one month) due to its water-soluble properties.
Sustained-release implants. The need for repeated intravitreal steroid injections has led to the development of sustained-release steroid implants. These devices include the nonbiodegradable FA implants Retisert, Iluvien, and Yutiq and the biodegradable DEX implant Ozurdex.
The risk of steroid response is higher with intravitreal FA implants. Bollinger et al. found that among patients who received an FA implant, 75% were prescribed topical glaucoma medications and 37% underwent filtration surgery.6 Similarly, Kiddee et al. found that up to 45% of those with FA implants required surgery.5 Most patients with ocular hypertension following a DEX implant can be managed medically, with only a small percentage (0.2%-3.2%) requiring glaucoma surgery.5
Clinical Course
Steroid-induced ocular hypertension typically occurs after several weeks of continued steroid treatment; however, an acute rise in IOP within hours has been reported. IOP elevation can occur within weeks with potent steroids or after several months with less potent forms. After four to six weeks of topical ocular steroids, 4% to 5% of patients have an IOP response greater than 16 mm Hg, one-third of patients have an increase of 6 mm Hg to 15 mm Hg (moderate steroid responders), and two-thirds of patients have no significant steroid response. Those with glaucoma have an increased risk of steroid response, as illustrated by Becker and Mills’ study, in which the mean IOP elevation in those with glaucoma was 17 mm Hg compared with 4 mm Hg in the control group.1 Upon cessation of steroids, IOP usually normalizes within one to four weeks.
Steroid-induced glaucoma develops if ocular hypertension persists and leads to progressive optic nerve damage. Clinically, steroid-induced glaucoma is very similar to POAG in presentation, aside from the significant history of steroid use. Patients present with elevated IOP, open angle on gonioscopy, optic nerve damage, and characteristic visual field changes. Adults and older children are usually asymptomatic, while young children may present with symptoms similar to primary infantile glaucoma (tearing, photophobia, blepharospasm). Steroid response can be more aggressive in infants and young children, with earlier onset of response and greater severity of glaucoma on presentation with signs of megalocornea and buphthalmos.
Management
The best way to manage steroid-induced glaucoma is to prevent its occurrence, if possible. It is important to review a patient’s medication list and history to assess the risk of steroid response. The ophthalmologist should use steroids judiciously and avoid or reduce their use in patients who have glaucoma or are glaucoma suspects. If steroids are required, they should be prescribed at the lowest efficacious dose and administered by the safest route.
Monitoring. IOP should be determined at baseline before steroids are started and then measured every few weeks after initiation of treatment, as glaucoma can develop at any time.
Medical management. If a patient develops steroid response, steroids should be discontinued or minimized as soon as possible. Ocular hypertension usually resolves within four weeks. Unfortunately, in about 3% of cases, the steroid response is irreversible.
If the underlying disease requires treatment with continued steroids, the physician may consider using a different, less potent steroid such as fluorometholone 0.1% or rimexolone 1%. NSAIDs can also be substituted in certain situations. If ocular hypertension occurs in response to systemically administered steroids, steroid-sparing agents should be considered.
If steroids cannot be discontinued, topical glaucoma medications, specifically aqueous suppressants, are used as the first-line agents. Prostaglandin analogues are another option for decreasing IOP, but they are relatively contraindicated in certain inflammatory conditions. If needed, oral acetazolamide is an effective temporizing therapy. Excision of the steroid depot or vitrectomy for intravitreal steroids may be needed.
Laser therapy. Apart from medical management, there is growing evidence that laser trabeculoplasty may be an effective alternative therapy or bridging treatment to incisional surgery for IOP reduction. Prior studies have shown variable success rates with argon laser trabeculoplasty, but more recent studies have demonstrated that selective laser trabeculoplasty can provide a rapid and substantial reduction in IOP. A recent retrospective study by Maleki et al. in patients with quiescent uveitis and steroid-induced glaucoma reported a 50% IOP reduction at one year.7
Surgical treatment. Incisional surgery may be indicated if the IOP is markedly elevated, if there is progressive optic nerve damage or visual field loss, or if a patient requires long-term steroid treatment. Most patients receive either a glaucoma drainage device or trabeculectomy for medically uncontrolled glaucoma.
Overall, surgical management results in adequate control of IOP. For patients with steroid-induced glaucoma who underwent trabeculectomy, Iwao et al. reported surgical failure in 11.9% to 16.7% at the three-year follow-up.8 A recent study in eyes with retinitis pigmentosa requiring long-term steroids for macular edema showed complete success in 77% of patients after Ahmed glaucoma valve (AGV) implantation at a mean follow-up of 38 months. There were no cases of failure in this study.9 For patients requiring steroid depot, Malone et al. found that AGV combined with an FA implant in chronic, severe, posterior uveitis and ocular hypertension is safe and effective.10 Goniotomy and canaloplasty have also been shown to be effective treatments for steroid-induced glaucoma.11,12 Cyclodestructive procedures are reserved for refractory cases.
___________________________
1 Becker B, Mills DW. JAMA. 1963;185(11):884-886.
2 Kersey JP, Broadway DC. Eye (Lond). 2006;20(4):407-416.
3 Cantrill HL et al. Am J Ophthalmol. 1975;79(6):1012-1017.
4 Korenfeld MS et al. J Cataract Refract Surg. 2009;35(1):26-34.
5 Kiddee W et al. Surv Ophthalmol. 2013;58(4):291-310.
6 Bollinger KE et al. Curr Opin Ophthalmol. 2009;20(2):99-103.
7 Maleki A et al. Ophthalmology. 2016;123(12):2630-2632.
8 Iwao K et al. Am J Ophthalmol. 2011;151(6):1047-1056.e1.
9 Eksioglu U et al. Int Ophthalmol. 2018;38(5):1833-1838.
10 Malone PE et al. Am J Ophthalmol. 2010;149(5):800-806.e1.
11 Choi EY, Walton DS. J Pediatr Ophthalmol Strabismus. 2015;52(3):183-188.
12 Brusini P et al. J Clin Med. 2018;7(2):31.
___________________________
Dr. Horan is an ophthalmology resident, and Dr. Salim is professor of ophthalmology, vice chair for Clinical and Academic Affairs, and director of the Glaucoma Service. Both are at the New England Eye Center of Tufts University School of Medicine in Boston. Financial disclosures: Dr. Horan: None; Dr. Salim: Aerie Pharmaceuticals: C,L.
See the disclosure key at www.aao.org/eyenet/disclosures.
___________________________
WRITE A PEARLS ARTICLE. For more information, see the writers guidelines at aao.org/eyenet/write-for-us.