By Chee Wai Wong, MMed(Ophth), Ian Yeo, FRCOphth, and Gemmy Cheung, FRCOphth
Edited By: Sharon Fekrat, MD, and Ingrid U. Scott, MD, MPH
Download PDF
Submacular hemorrhage (SMH) is an uncommon complication of choroidal or retinal vascular abnormalities, including choroidal neovascularization (CNV), polypoidal choroidal vasculopathy (PCV), and retinal macroaneurysm. Of these, PCV is the condition most frequently associated with large SMH (reported in 20%-63% of eyes with PCV).
SMH can damage photoreceptors as a result of iron-induced toxicity, with irreversible retinal injury occurring as early as 24 hours after onset of the hemorrhage. Only 11% of eyes with SMH achieved a best-corrected visual acuity (BCVA) better than 20/200 after 2 years of observation.1 Avery et al. found a mean loss of 3.5 lines of VA after 3 years in eyes with subfoveal hemorrhage secondary to exudative age-related macular degeneration (AMD), and almost half of these eyes (44%) had lost 6 or more lines.2 The presence of subretinal CNV membranes predicts poorer final visual acuity.3
Diagnosis
Patients often present with decreased central vision, sometimes 20/200 or worse. On dilated fundus examination, SMH can be observed as an elevation of the neurosensory retina, which can also be associated with a hemorrhagic detachment of the retinal pigment epithelium (RPE).
Subretinal versus sub-RPE. It is important to distinguish subretinal blood from sub-RPE blood, as hemorrhage at the subretinal level may be more harmful to photoreceptors. Clinically, subretinal blood may appear bright red, while sub-RPE blood appears darker. Optical coherence tomography (OCT) is a useful imaging tool for distinguishing the level at which hemorrhage has occurred. Yellowish-white depigmented blood or vitreous hemorrhage may also be present.
Seeking the cause. Although the underlying cause may be apparent on clinical examination, further imaging is often required to elucidate it. If the ocular media are sufficiently clear, fundus imaging should be performed with fluorescein angiography (FA) and indocyanine green angiography (ICGA) to identify and locate the primary pathology to guide treatment.
Treatment
Several monotherapy or combined approaches are used, including the following:
- Anti–vascular endothelial growth factor (anti-VEGF) monotherapy
- Pneumatic displacement (PD) + anti-VEGF therapy
- Intravitreal recombinant tissue plasminogen activator (rtPA) + anti-VEGF + PD
- Pars plana vitrectomy + subretinal injection of rtPA + subretinal or intravitreal PD
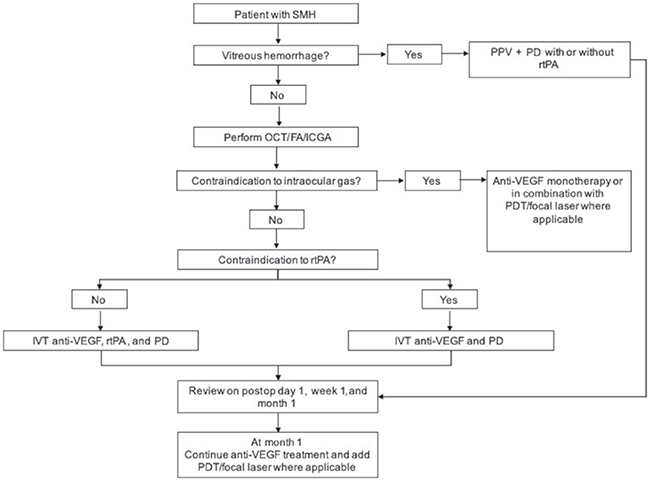 |
|
TREATMENT ALGORITHM. Abbreviations: FA, fluorescein angiography; ICGA, indocyanine angiography; IVT, intravitreal; OCT, optical coherence tomography; PD, pneumatic displacement; PDT, photodynamic therapy; PPV, pars plana vitrectomy; rtPA, recombinant tissue plasminogen activator; SMH, submacular hemorrhage; VEGF, vascular endothelial growth factor.
|
Anti-VEGF Therapy
Anti-VEGF monotherapy is a viable option for the treatment of SMH secondary to neovascular AMD or PCV. Studies evaluating anti-VEGF monotherapy have demonstrated robust visual outcomes, with 44% to 60% of eyes achieving 3 or more lines of VA improvement at 6 months.4 One study showed that although eyes with thick SMH (>450 μm) achieved better visual outcomes with combination therapy, thinner SMH could be managed as effectively with anti-VEGF monotherapy.5
Choice of anti-VEGF. There is no evidence to suggest the superiority of one anti-VEGF agent over another in treating SMH. However, it should be noted that aflibercept has been shown to be cleaved by rtPA-induced plasmin in vitro, which might reduce the antiangiogenic effect of aflibercept when combined with intravitreal rtPA for the treatment of SMH.
Pneumatic Displacement
PD utilizes intravitreal injection of an expansile gas to move blood away from the fovea. The procedure can be performed with or without intravitreal rtPA but is usually combined with intravitreal anti-VEGF therapy to treat the underlying pathology.
PD has been shown to be effective in displacing SMH, even without use of rtPA; for example, complete displacement was achieved in 80% of eyes within a week of treatment.1 Moreover, it provides the added benefit of faster visual recovery compared with anti-VEGF therapy alone for the treatment of SMH secondary to PCV.1
Adjunctive effects. By clearing thick blood away from underlying CNV membranes or polyps, PD can potentially enhance the action of anti-VEGF drugs. In eyes with PCV, displacement of blood allows the physician to view the underlying polyps with ICGA, thus facilitating treatment with either focal laser or photodynamic therapy (PDT).
Clinical considerations and caveats. Some important considerations should be discussed with the patient before proceeding with PD.
Positioning. Is the patient able to maintain the recommended facedown position for prolonged periods over several days?
Cataract. In phakic patients, PD can hasten the development or progression of cataract.
Intraocular pressure. Elevation of IOP can occur in the ensuing days and up to 1 or 2 weeks after the procedure, depending on the gas injected. Thus, IOP should be monitored closely, and PD should be used with caution in patients with preexisting glaucoma.
Location of SMH. The gas can inadvertently shift more subretinal blood toward the fovea, especially if most of the hemorrhage lies in the superior macula.
How to perform PD. This procedure can be performed in the outpatient setting in a clean room under sterile conditions and topical anesthesia. If rtPA or anti-VEGF therapy is planned, these agents should be administered prior to gas injection.
Either SF6 or C3F8 gas can be used. The gas is drawn into a 3-mL syringe without dilution and injected with a 25-gauge needle via the pars plana into the vitreous cavity. After the injection, VA should be assessed with counting fingers, and anterior chamber paracentesis is performed as required.
After treatment. The patient is advised to remain in a facedown position as much as possible for a few days. The patient should return the day after PD for a dilated fundus examination and IOP check, with similar follow-up occurring at 1 week and at monthly intervals thereafter, depending on subsequent treatment.
Recombinant Tissue Plasminogen Activator
rtPA is an enzyme that catalyzes the conversion of plasminogen to plasmin, the main enzyme involved in clot breakdown. Several studies evaluating injection of rtPA combined with PD have reported visual acuity gain of 3 lines or more in 42% to 66% of eyes.6
It can be administered as a subretinal injection or injected into the vitreous cavity for the lysis of submacular blood clots. Intravitreal injection of rtPA is the less invasive, less time consuming, and less technically challenging of these approaches. Studies have confirmed that rtPA injected intravitreally can migrate across the vitreous cavity and the retina into the subretinal space.7
Reported rates of complete SMH displacement and short-term visual outcomes are similar between subretinal injection of rtPA and intravitreal rtPA with PD.8
Adverse effects of rtPA. Ocular side effects of rtPA include photoreceptor cell loss, RPE pigmentary changes, and exudative retinal detachment. These effects appear to be dose dependent, and a dosage of less than 25 μg/0.1 mL is recommended to avoid them. Also, injection of rtPA into a gas-filled eye (which concentrates the drug at the retinal surface) and repeat injections should be avoided.
Hemorrhagic. rtPA can cause hemorrhagic complications, an important consideration if rtPA is to be given within 72 hours of bleeding onset. (It should be noted, however, that breakthrough vitreous hemorrhage can also occur regardless of the treatment, and patients should be made aware of this during the informed consent process.)
Although there have been no reports of systemic side effects with these low intraocular doses, the possibility of systemic hemorrhagic complications should not be forgotten, especially in susceptible patients, such as those on anticoagulants.
Vitrectomy
If vitreous hemorrhage is present, pars plana vitrectomy facilitates its removal, which improves fundus visualization for monitoring treatment response and allowing subretinal injection of rtPA. The procedure usually involves a combination of small-gauge vitrectomy, subretinal injection of rtPA using a 41-gauge flexible cannula, and treatment of the underlying pathology with laser or anti-VEGF, followed by fluid-air exchange and intravitreal gas tamponade with nonexpansile SF6 or C3F8.
Subretinal PD. Subretinal PD, in which air is injected into the subretinal space, has been described as an alternative to PD with intravitreal gas. The higher pressure exerted by subretinal air may be more effective in displacing the subretinal blood clot after rtPA-assisted clot lysis compared with intravitreal gas tamponade.
Subretinal PD eliminates the need for prolonged facedown positioning and the risk of gas-related IOP elevation, but it may be associated with higher risk of macular hole formation.9, 10
Study results. In a review of 38 studies, Van Zeeburg et al. found no clear difference in complete displacement of SMH or complication rate between vitrectomy with subretinal injection of rtPA versus intravitreal rtPA with PD without vitrectomy.11
Hirashima et al. reported the results of rtPA-assisted vitrectomy, gas tamponade, and postoperative treatment with intravitreal ranibizumab or PDT, demonstrating a visual improvement of 3 lines or more in 66% of eyes.12 These results compared favorably with other groups using a similar surgical technique.13-15
Possible downside. A potential disadvantage of vitrectomy is the rapid washout of anti-VEGF agents in vitrectomized eyes, which may necessitate more frequent intravitreal injections in patients with CNV or PCV.
Management of the Underlying Pathology
FA, ICGA, and OCT angiography are essential imaging modalities in diagnosing the underlying cause of SMH and in selecting and monitoring the subsequent treatment.
Macroaneurysms. These vascular abnormalities can be adequately managed with focal thermal laser photocoagulation.
CNV. Intravitreal anti-VEGF remains the gold standard for treatment of CNV.
PCV. Management of PCV depends on its location. Subfoveal and juxtafoveal PCV can be treated with anti-VEGF as monotherapy or in combination with PDT. Combined therapy may help to quickly close the polypoidal lesions and facilitate resolution of SMH, but it carries the risk of RPE tear. Defer-ring PDT until most of the blood has resorbed allows better visualization of underlying polyps and reduces attenuation of laser energy.
Extrafoveal PCV can be managed effectively with anti-VEGF in combination with either focal thermal laser photocoagulation or PDT.
Conclusions
As a general approach to SMH treatment, PD can be combined with intravitreal rtPA if there are no contraindications. In addition, anti-VEGF therapy should be administered as indicated by the underlying pathology. Although SMH can be challenging to manage, reasonable visual outcomes can be achieved with timely and appropriate intervention.
___________________________
1 Wong CW et al. Prog Retin Eye Res. 2016;53:107-139.
2 Avery RL et al. Retina. 1996;16(3):183-189.
3 Berrocal MH et al. Am J Ophthalmol. 1996;122(4):486-493.
4 Cheung CM et al. Graefe’s Arch Clin Exper Ophthalmol. 2013;251(1):19-25.
5 Shin JY et al. Am J Ophthalmol. 2015;159(5):904-914 e901.
6 Kitagawa Y et al. Ophthalmology. 2016;123(6):1278-1286.
7 Hesse L et al. Retina. 2000;20(5):500-505.
8 van Zeeburg EJ et al. Graefe’s Arch Clin Exper Ophthalmol. 2013;251(3):733-740.
9 Martel JN, Mahmoud TH. JAMA Ophthalmol. 2013;131(12):1632-1635.
10 Kadonosono K et al. Ophthalmology. 2015;122(1):123-128.
11 van Zeeburg EJ, van Meurs JC. Ophthalmologica. 2013;229(1):1-14.
12 Hirashima T et al. Retina. 2015;35(10):1969-1978.
13 Chang W et al. Am J Ophthalmol. 2014;157(6):1250-1257.
14 Kapran Z et al. Ophthalmic Surg Lasers Imaging Retina. 2013;44(5):471-476.
15 Papavasileiou E et al. Retina. 2013;33(4):846-853.
___________________________
Dr. Wong is a vitreoretinal fellow, Dr. Yeo is a senior consultant and head of the medical retina department, and Dr. Cheung is a senior consultant and deputy head of the medical retina department; all are at the Singapore National Eye Centre. Relevant financial disclosures: None.