Download PDF
Presumed ocular histoplasmosis syndrome (POHS) is a chorioretinal disease that is asymptomatic in the majority of cases. However, the condition may progress to peripapillary atrophy, chorioretinal lesions, choroidal neovascularization (CNV), disciform macular scars, and, ultimately, visual loss. In patients with CNV, the mainstay of treatment is anti-VEGF therapy, which can slow the progression of disease and offer significant benefit. Although the pathogenesis of POHS remains to be fully elucidated, it is believed to be caused by the dimorphic fungus Histoplasma capsulatum.
Epidemiology
POHS is an important cause of visual loss among individuals living in areas endemic for H. capsulatum, including the Mississippi and Ohio River valleys.The fungus is typically carried by chickens and pigeons and is also found in bat droppings.
The presumed culprit: H. capsulatum. There is no confirmed direct relationship between H. capsulatum and the ocular syndrome, leading to the name presumed ocular histoplasmosis syndrome. However, epidemiologic and histologic data strongly suggest a causal relationship. For example, the majority of patients diagnosed with POHS in the United States have at some point lived in an endemic area, and histoplasmin skin testing is positive more often in those with the syndrome than in controls. In addition, DNA from H. capsulatum has been isolated from an enucleated eye diagnosed with POHS and from the peripheral blood of a patient with acute ocular histoplasmosis.
Who is affected. POHS affects males and females equally, typically between the second and fifth decades of life, and the majority of disciform lesions occur in Caucasians.
Risk factors for CNV. Smoking is the most significant risk factor associated with the development of CNV in POHS; other risk factors include older age and lower educational level.
Genetic predisposition. Although the mechanism underlying CNV initiation in POHS is unclear, studies suggest a link between histocompatibility anti-gens and the development of disciform lesions. POHS was found to be associated with alleles in the HLA-DR and HLA-DQ loci. Specifically, there is a significant association between the HLA-DR15 and HLA-DQ6 haplotype and CNV in POHS.1 These findings suggest an underlying genetic predisposition for the development of POHS.
Pathophysiology
The inciting event in POHS is thought to be inhalation of H. capsulatum, with subsequent systemic infection occurring through hematogenous dissemination. Initial infection with histoplasmosis may be asymptomatic, or it can cause a benign illness with flu-like respiratory symptoms, typically in childhood. Therefore, most patients diagnosed with POHS have no known history of systemic infection with H. capsulatum.
Once systemic infection occurs, the fungus can travel to the eye and cause focal infection in the choroid. The en-suing choroiditis, marked by lymphocytic infiltration, can either subside subclinically or leave an atrophic scar. This atrophic scarring can disrupt the Bruch membrane, the choriocapillaris, and the retinal pigment epithelium. Subsequently, CNV occurs, leading to hemorrhage and exudative leakage. Over time, the result is loss of macular function and the development of disciform macular scars, which can lead to loss of central vision (Fig. 1).
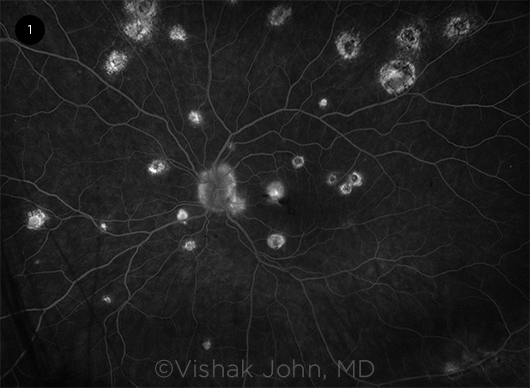 |
|
CNV. Fluorescein angiogram depicting staining of macular scars and leakage in the choroidal neovascular membrane of the left macula.
|
Clinical Features
The four main signs of POHS are “punched-out” chorioretinal scars, called histo spots; peripapillary atrophy; CNV, which can be classified as subfoveal, extrafoveal, or juxtafoveal; and the absence of vitritis or anterior chamber inflammation. These features can be seen and diagnosed on funduscopic exam (Fig. 2). The clear vitreous is an important diagnostic criterion, as it helps differentiate POHS from multifocal choroiditis.
Histo spots are white lesions with variable pigmentary changes that appear in the macula or periphery and are typically smaller in size than the optic disc. Patients can present with multiple histo spots, which are often bilateral and which can continue to increase in size and number over time. Because these spots are largely asymptomatic, patients rarely present at this stage.
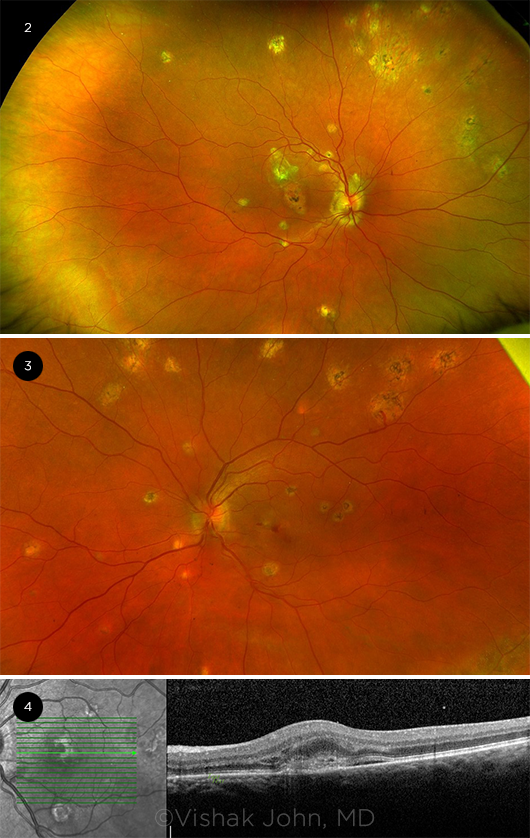 |
|
IMAGING. (2) Peripapillary atrophy and punched-out scars in the periphery. (3) Active choroidal neovascular membranes with bleeding in the macula secondary to POHS. (4) Optical coherence tomography showing choroidal neovascular membranes with subretinal fluid and intraretinal fluid in the left eye.
|
Diagnosis
POHS may be diagnosed early by the incidental discovery of asymptomatic histo spots or at a later stage, when the patient experiences significant visual loss secondary to macular CNV (Fig. 3).
Imaging. In addition to funduscopic examination, fluorescein angiography (FA; Fig. 1) and spectral-domain optical coherence tomography (SD-OCT; Fig. 4) can be used to identify the location of CNV in patients presenting for visual loss. On FA, CNV typically presents with radiating areas of hyperfluorescence, whereas hypofluorescence can be an indication of associated subretinal hemorrhage.
SD-OCT can provide additional information about the extent of CNV and can be used to monitor disease progression. On SD-OCT, histo spots may appear as a focal area of outer retinal atrophy, corresponding to their description as “punched out.” The normal outer hyperreflective bands of the retina may also lose their internal reflectance and appear disorganized.
Disease Course and Prognosis
Even if diagnosed at an early stage that does not warrant intervention, patients with POHS need to be followed over time because of the potential for CNV formation and macular scarring. The most important risk factor for visual loss is proximity of CNV to the foveal avascular zone (FAZ).2 Prognostic factors associated with positive visual outcomes include extrafoveal lesions, lack of subretinal hemorrhage, and small areas of CNV.2
Because of its ability to distinguish lesion morphology, OCT is an important tool in managing POHS. It is useful in detecting subretinal fluid, monitoring treatment response, and evaluating the need for further anti-VEGF therapy in patients with POHS.3
Treatment
Because CNV secondary to POHS causes hemorrhage, disciform lesions, and subsequent vision loss, the goal of therapy centers around obliterating the neovascular complexes. Several treatment approaches have been used to manage such CNV, including laser photocoagulation, photodynamic therapy (PDT), and anti-VEGF intravitreal injections, now the most common treatment.
Anti-VEGF therapy. As with other causes of CNV such as age-related macular degeneration and retinal vein occlusion, anti-VEGF therapy has become the mainstay of treatment for CNV secondary to POHS. However, no anti-VEGF agent is currently approved by the FDA for a POHS indication. Several studies have looked at the use of bevacizumab, ranibizumab, and aflibercept in the treatment of POHS.4-7
In a study analyzing 24 treatment-naive eyes with CNV from POHS, patients given an average of 6.8 bevacizumab injections per year demonstrated improved visual acuity (VA) from an average of 20/150 at baseline to 20/45 at 12 months, with no significant complications. Additionally, 58% of treated eyes had VA of 20/40 or better at 12 months compared with 21% of eyes at baseline.4
The HANDLE study investigated the effect of dosing frequency of intravitreal aflibercept for POHS-related CNV. At 12 months, no statistically significant difference in visual or anatomic outcomes was detected between the group on a sustained-dosing regimen (average of 7.5 doses) compared with the group receiving as-needed therapy (average of 4.6 doses).5
Combination therapy with anti-VEGF agents and PDT has also been studied, but retrospective analysis did not show a statistically significant difference in VA between patients who received intravitreal bevacizumab monotherapy and those who received combination therapy.7
Laser therapy. The efficacy of laser photocoagulation for the treatment of extrafoveal and juxtafoveal CNV lesions secondary to POHS was initially demonstrated by the Macular Photocoagulation Study (MPS) Group. Specifically, for extrafoveal lesions, defined as having their foveal edge 200 to 2,500 μm from the FAZ center, 68% of eyes treated with argon laser photocoagulation had VA of 20/40 or better at five-year follow-up, compared with 43% in untreated eyes.8
Based on the findings from the MPS trials, laser photocoagulation was determined to be an effective and safe treatment for extrafoveal and juxtafoveal CNV due to POHS (subfoveal lesions are poor candidates for photocoagulation). However, this study was conducted prior to the advent of anti-VEGF therapy. Laser therapy can cause scarring, and over time these scars have the potential to expand. Thus, anti-VEGF therapy is considered the more efficacious and safe treatment option.
Photodynamic therapy. PDT has been established as a suitable treatment for juxtafoveal and subfoveal CNV in POHS and is FDA approved for this indication. It involves intravenous injection of a photosensitizing drug that circulates within the CNV membrane. At peak absorption, nonthermal light can be used to irradiate the CNV membrane, causing thrombosis and closure.
Retrospective studies have demonstrated that among patients who received PDT for juxtafoveal lesions, 80% demonstrated improved or stable VA, with 60% displaying VA of 20/40 or better at follow-up. Similarly, 83.3% of patients with subfoveal lesions had improved or stable VA at follow-up, with 50% demonstrating VA of 20/40 or better.9
Surgical Options
Although submacular surgery was formerly an important treatment for patients with significant vision loss from subfoveal CNV secondary to POHS, it has been superseded by PDT and anti-VEGF therapy. One major problem with surgical treatment is the significant rate of CNV recurrence, which is higher than the rate following photocoagulation.10 In fact, the Submacular Surgery Trials demonstrated that there was no statistically significant difference in outcomes and visual improvement at 24 months between patients who had submacular surgery and those who were simply observed.11
At the time of these trials, neither PDT nor anti-VEGF agents were available. Because of its associated risks and the emergence of superior treatment options, submacular surgery is no longer used.
Conclusion
Routine ophthalmic exams and close monitoring of patients with signs of POHS can allow for early diagnosis and intervention to avert serious visual impairment. In addition, the advent of effective therapies, such as anti-VEGF agents and PDT, has made it possible to delay disease progression, restore vision, and improve quality of life in even the most severe cases.
__________________________
1 Dabil H et al. Hum Immunol. 2003;64(10):960-964.
2 Chandra SR, Meyer MD. Presumed ocular histoplasmosis syndrome. In: Nema HV, Nema N, eds. Gems of Ophthalmology: Diseases of Uvea. Jaypee; 2018:78-92.
3 Liu TYA et al. Case Rep Ophthalmol Med. Special Report. 2018:Article ID 4098419.
4 Ehrlich R et al. Retina. 2009;29(10):1418-1423.
5 Toussaint BW et al. Retina. 2018;38(4):755-763.
6 Ramaiya KJ et al. Ophthalmic Surg Lasers Imaging Retina. 2013;44(1):17-21.
7 Cionni DA et al. Ophthalmology. 2012;119(2):327-332.
8 Macular Photocoagulation Study Group. Arch Ophthalmol. 1991;109(8):1109-1114.
9 Liu JC et al. Retina. 2004;24(6):863-870.
10 Melberg NS et al. Ophthalmology. 1996;103(7):1064-1068.
11 Hawkins BS et al; Submacular Surgery Trials Research Group. Arch Ophthalmol. 2004;122(11):1597-1611.
__________________________
Dr. John is a vitreoretinal surgeon at Vistar Eye Center and an assistant professor of surgery at the Virginia Tech Carilion School of Medicine in Roanoke, Va. Financial disclosures: Genentech: C; Regeneron: C. Dr. Shah is a PGY1 internal medicine resident at Harbor-UCLA Medical Center in Los Angeles. Financial disclosures: None.
See the disclosure key at www.aao.org/eyenet/disclosures.