Download PDF
Four experts weigh in on what to consider when helping patients decide on their course of treatment.
Once thought to be very different from one another, vitreomacular traction (VMT) syndrome, macular hole, and some macular puckers are now understood to be manifestations of the same fundamental problem: anomalous posterior vitreous detachment (PVD) with persistent vitreomacular adhesion (VMA). Moreover, this problematic combination of PVD and VMA is also associated with retinal tears and detachment, diabetic retinopathy (DR), and exudative age-related macular degeneration (AMD).1
Although there have been many advances in the treatment of these vitreomacular interface diseases, viewpoints differ on the best approaches, particularly the current role of pharmacologic vitreolysis.2
Anomalous PVD and Symptomatic VMA
“We are all born with a vitreous gel that is optically clear and 100 percent gel, but the process of the gel liquefying over time is life-long,” said Nancy M. Holekamp, MD, at the Pepose Vision Institute in St. Louis. “When we’re 100 years old, it’s 100 percent liquid and full of floaters.”
As a gel, the vitreous adheres to the retina everywhere on its surface, like Velcro. “By our mid-60s, enough of the gel has liquefied to start sloshing around in the eye, with the remaining solids pulling on the retina,” Dr. Holekamp explained. At the same time, there is a weakening of the “Velcro” between the posterior vitreous cortex and the retina. When this two-step process is synchronized, as it is in most people, the vitreous pulls free of the retina, typically causing floaters, and that’s the end of the story. This is called posterior vitreous detachment, or PVD.
Pathophysiology. In some instances, the vitreous gel has liquefied, but the adhesion of the vitreous to the macula (VMA) has not weakened, resulting in anomalous PVD. The vitreous can split (partial-thickness PVD), creating traction on the retina. When traction occurs in the macula, VMT syndrome can occur (as well as exacerbation of DR and AMD). If the direction of tangential traction is outward, a macular hole may form, explained Jerry Sebag, MD, at the VMR Institute and the Doheny Eye Institute in California. If traction goes inward, macular pucker may result.3,4
The consequent structural changes in the macula can cause metamorphopsia, blurred vision, central visual field defects, and image size disparity. About 1.5 percent of the population is estimated to have vitreomacular interface disease, although that figure is expected to rise with the growing number of older people and more widespread use of optical coherence tomography (OCT).5
It is worth noting that macular pucker is different from VMT. The latter has anomalous PVD with separation peripherally but full-thickness vitreous cortex pulling on the macula, usually in an axial or oblique direction. Macular pucker results from anomalous PVD with vitreoschisis, which is a split in the posterior vitreous cortex that leaves the outermost layer of vitreous attached to the macula while the rest of the vitreous cortex detaches away from the retina.1 There can also be minor damage to the retina, stimulating an immune response. The subsequent proliferation of cells in the macular area can form a layer of scar tissue that tightens, creating traction on the macula and causing it to pucker. Although a pucker occasionally disintegrates (particularly in people under age 50), the condition is permanent in the majority of patients.
Terminology confusion. There is no universally accepted nomenclature or classification system for vitreomacular interface diseases. “It’s difficult to compare outcomes when we’re defining things differently from one another,” said John T. Thompson, MD, at the Wilmer Eye Institute in Baltimore. “Other than with OCT pictures, there’s no way to accurately communicate a patient’s condition.”
Just listing some of the terms used for macular pucker highlights the problem: epiretinal membrane (ERM), epimacular membrane, preretinal membrane, cellophane maculopathy, and retinal wrinkle, for instance. Andreas K. Lauer, MD, at Oregon Health & Science University in Portland, uses the term epiretinal membrane when talking with colleagues and macular pucker when talking with patients, given that the Academy’s patient education materials use macular pucker. Dr. Sebag advocates use of the term premacular membrane rather than ERM because it is more specific, and he prefers to use macular pucker to describe the effects of the premacular membrane on the macula.
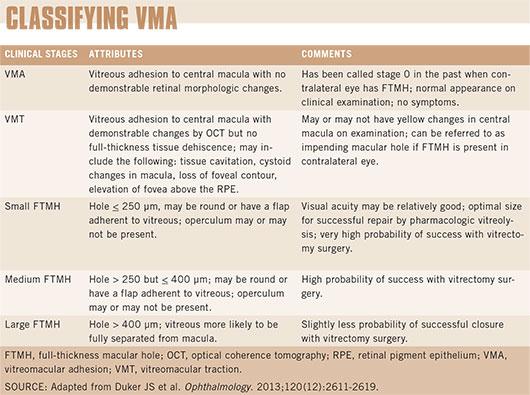
There are a few classification systems floating around, according to Dr. Thompson, but the most recent is an OCT-based anatomic classification system proposed by the International Vitreomacular Traction Study (IVTS) Group6 (see "Classifying VMA"). Given the current level of understanding and our diagnostic capabilities, this proposed classification system is expected to help clinicians select the most appropriate treatment and is potentially useful in the execution and analysis of clinical studies, the experts agreed.
A Word of Caution
The most important symptom to watch for in vitreomacular interface diseases is metamorphopsia. The most important physical finding is evidence of VMA with disruption in the macula. “Just having VMA [with no disruption] can be normal,” said Dr. Holekamp. But as Dr. Sebag pointed out, “When a patient has VMA with structural changes in the macula, that’s VMT.” Depending on the severity of symptoms and the OCT images, referral to a retina specialist is likely indicated. |
Treatment Options and Considerations
Thanks to advances in imaging technologies, clinicians are identifying vitreomacular interface diseases earlier than ever before. And the fact that mild cases can now be picked up by OCT raises questions about treatment. Although surgery remains the standard of care, ophthalmologists may wonder if they should try something else first. “The answer is largely driven by symptoms and visual acuity,” said Dr. Thompson.
“As physicians, we have to collect data before making a treatment decision. We look at vision, look at the OCT scan, and talk to patients about how much they’re bothered by symptoms,” said Dr. Holekamp, who emphasized three points: 1) “We don’t treat scans; we treat people. Just because we see an awful-looking OCT doesn’t mean a patient needs intervention if his or her vision is good.” 2) “Most epiretinal membranes [macular puckers] don’t get worse. Only a minority of patients with epiretinal membranes go on to require intervention.” 3) “These are mechanical problems, so they need mechanical solutions. If intervention is appropriate, the options are either pharmacologic vitreolysis or surgery.”
Watchful Waiting
When to watch. When there is evidence of disruption to the macula on the OCT scan but a patient is asymptomatic, Dr. Lauer recommends watchful waiting. He gives these patients an Amsler grid to monitor their status at home and schedules them for a follow-up dilated exam about six months later. “Only when patients are symptomatic and are willing to accept the risks of intervention do I treat vitreomacular interface diseases.” Similarly, Dr. Holekamp watches patients with VMT or ERM if they have good vision, if their OCT has only mild changes, or if they simply aren’t bothered by it.
When not to watch. However, Dr. Holekamp never watches anyone with a macular hole. “Ten out of 10 retinal specialists would recommend treatment for macular hole,” she said. “Most would say vitrectomy; some might say pharmacologic vitreolysis.”
And Dr. Sebag argued that watchful waiting is no longer an option. “We now have pharmacologic vitreolysis, which can be administered while waiting. If it works, great; if not, we can always move on to surgery. My preferred choice is to inject and then wait.”
Pharmacologic Vitreolysis
Historically, treatment for symptomatic VMA has been surgical. But in January 2013, when the vitreolytic agent ocriplasmin (Jetrea) came to market, the era of pharmacologic vitreolysis began.
Just as in surgery, the objective of pharmacologic vitreolysis is to relieve traction on the macula by lysing the anomalous VMA. “Ocriplasmin makes sense when there is a small area of adhesion with VMT or a small or medium full-thickness macular hole with VMT,” said Dr. Thompson. In such cases, success rates are around 40 percent for good candidates, according to Drs. Thompson and Lauer; Dr. Holekamp’s estimate is 50 percent. At $4,000 per shot, cost is a major consideration, given that some retina specialists are still having reimbursement problems with ocriplasmin. (While the drug is cheaper than surgery, the success rate of surgery approaches 100 percent.)
Patient selection. Dr. Sebag, who was an investigator in the phase 2 and 3 trials of ocriplasmin, pointed out that when the trials reported 27.1 percent efficacy, that rate was for all participants. “We didn’t know that there were favorable characteristics at that time, but we were able to identify retrospectively certain features that incrementally increased the likelihood of success,” he said.
When the patient is under age 65, is phakic, does not have a macular pucker, and has an adhesion less than 1,500 µm, the probability of success goes up to 60 to 80 percent. “These criteria are not obligatory; they just help the physician and patient weigh the probability of success,” Dr. Sebag said, adding that the success of injecting ocriplasmin for a macular hole is directly related to the hole’s size. Data show that the drug works fairly well for holes 250 to 400 µm in diameter and does best for holes under 250 µm, he said. No holes larger than 400 µm have resolved with ocriplasmin to date.
Safety concerns. Clinical trial data showed ocriplasmin to be safe, but the postmarketing experience has uncovered some complications, including temporary separation of the retina from the retinal pigment epithelium, with disruption of the ellipsoid layer, and electrophysiological changes as detected by electroretinography. “It’s not a common phenomenon, but it’s something that needs to be studied more,” said Dr. Thompson.
Dr. Holekamp concurred. “Ocriplasmin is expensive and works under ideal circumstances only some of the time. Throw into the equation that there may be complications that weren’t described in the clinical trials but that we may now be seeing, and I say to myself that I have concerns. I’ll let our profession figure out the true risks and efficacy and then adjust my ocriplasmin use accordingly.”
In contrast, Dr. Sebag is of the opinion that the safety concerns are overblown. “Personally, I haven’t experienced any untoward events,” he said. “As a consultant to the company, I have been provided with data on all the adverse events that occurred in the study, and the transient nature of these adverse events and the minuscule number of people affected impressed me. It’s not something that I think should thwart our implementation of this approach. Of course, safety is of paramount importance; thus, we should collect our experiences to see how implementation in the real world compares to the clinical trials.”
Dr. Sebag further emphasized that ocriplasmin is the first pharmacologic vitreolysis drug brought to market. “There are going to be problems, just like there were for the first antibiotic when it was introduced. But it’s the right way to go. It’s going to help patients, lower health care costs, and be a wonderful new paradigm. As we learn more, we will refine our approach, and we’ll have better success rates.”
Current use. “We all became so excited when ocriplasmin came to market, but I’m more cautious now,” said Dr. Lauer. He considers using ocriplasmin in patients who are phakic, have a focal area of VMT, have no concurrent eye problems or history of previous retinal laser treatment, and are not diabetic. Two-thirds of his VMT patients come in asking for ocriplasmin, as they don’t want surgery and are eager to try an injection first. He has had success in a third of his patients.
Dr. Sebag said that his patients are similarly keen to try the drug. “Overwhelmingly, they choose the injection because they understand that they’re not compromising themselves—meaning, if it doesn’t work, we do the surgery, and the surgery proceeds as it would have if they’d never had the injection, with the same likelihood of success.” Like Dr. Lauer, Dr. Sebag estimated that it works in about one in three patients.
“With careful patient selection, some people can avoid surgery with ocriplasmin, and that’s a significant advance,” Dr. Thompson said. “It also might be helpful in the secondary complications that relate to VMT in patients with diabetic retinopathy and perhaps neovascular AMD. That would be invaluable.
(click to expand)
Surgery
In surgery, the vitreous is removed to gain access to the site of VMA so that the surgeon can remove the adhesion from the macula. Vitrectomy has been around for 40 years, but advances in surgical equipment and techniques have transformed it into a safer and more effective procedure.
Small-gauge vitrectomy. Today, many retina surgeons have adopted sutureless techniques with small-gauge instruments under local anesthesia. The surgical and postoperative recovery time has decreased as a result. If there’s one surgical tip that Dr. Lauer would give, it’s to not spend too much time in the eye. “I strongly recommend either 23- or 25-gauge instruments to reduce operating time, invasiveness, postoperative discomfort, and the risk of complications.”
ILM peeling. There is continuing debate for and against peeling the internal limiting membrane (ILM), with studies supporting both sides. “I make an effort to remove the ILM in all macular hole cases,” said Dr. Lauer. Dr. Sebag concurred, saying, “Studies have proven that ILM removal results in better outcomes in macular hole cases.”
In macular pucker, Drs. Lauer and Sebag tend to remove the premacular membrane only. “Sometimes, in the course of removing the epiretinal [premacular] membrane, there’s an adhesion between the two, and a part of the ILM will come with it. If there appears to be a partial peeling of the ILM, I may remove it all. But I do not deliberately remove the ILM in all epiretinal membrane cases. There are studies indicating that when you remove the ILM, there can be some injury to the ganglion cells,” said Dr. Lauer.7
Dr. Thompson is an advocate for peeling the ILM in VMT syndrome, ERM, and macular hole surgery almost all the time. “That way I’m assured I’ve removed the VMT,” he said. “Many patients with VMT have at least a mild degree of ERM; and, by peeling the ILM, I know I’ve removed the associated ERM that could potentially cause problems with VMT later on.”
Dr. Holekamp said that she selectively peels the ILM in medium-to-large macular holes and moderate-to-difficult macular pucker cases, but she usually does not do so in VMT cases.
Chromodissection. The challenge of membrane peeling can be reduced with chromodissection—the staining of membranes to facilitate their removal. “Peeling the ILM can be difficult, so my advice is to use indocyanine green [ICG] stain,” said Dr. Thompson. “It can be helpful in making certain that you’ve removed the ILM successfully. Some surgeons prefer to dust the ILM with triamcinolone, which is a perfectly good method, but the triamcinolone only shows you where you’ve removed the ILM; it doesn’t allow you to see the ILM to grab it as easily.” Dr. Sebag noted, “I believe that ICG alters the ILM on a molecular level, making it easier to peel.”
Dr. Lauer uses ICG for macular hole surgery to visualize the ILM. But because some studies have raised the specter of ICG toxicity, he uses trypan blue or triamcinolone for macular pucker and VMT. Dr. Holekamp simply uses triamcinolone for everything.
Gas tamponade. “For VMT and ERM, gas tamponade is not needed, but if a surgeon feels it’s necessary, two to three days is adequate,” said Dr. Thompson.
For macular holes, gas tamponade to temporarily seal off the hole is the standard of care. Most surgeons use a short-acting tamponade, such as sulfur hexafluoride (SF6), although some surgeons are now using even shorter-acting agents, such as sterile air. Early research has reported similar rates of hole closure between air and SF6 and a reduction in the time patients needed to spend in the face-down position.8 However, evidence is currently inadequate, and air tamponade is not recommended.
To help ensure that the gas bubble covers the macular hole, Dr. Thompson prescribes seven to 10 days of face-down positioning after surgery, but this practice is still up for debate (see "Face Down—or Not?").
Face Down—or Not?
Retina specialists have yet to come to a consensus on whether the traditional recommendation of face-down positioning after macular hole surgery should be followed or abandoned.
Dr. Thompson noted that some surgeons are advocating no (or very short periods of) face-down positioning, “and the reported success rates are pretty good—but the success rates are a little better if the patient stays face down.” He added, “There are some OCT devices where you can image through the gas bubble, and studies have shown that about two-thirds of macular holes close within one to two days; but one-third don’t close, even up to a week after surgery. So if you want to catch all-comers, then it’s useful to have the gas bubble against the macula for a week or even longer.”
Dr. Lauer counts himself among the group of surgeons moving away from face-down positioning. “I’m not very strict about it. When I can get a good gas fill in patients who’ve already had cataract surgery, I simply ask them to lie on their sides at night and remain upright during the day. Typically, the macular hole will heal in the first week. In phakic patients, I encourage them to do face-down positioning to reduce the degree of cataract formation, but I’m not overly compulsive about it. I am compulsive about face-down positioning if there’s not a good enough gas fill after surgery; likewise for large macular holes,” he said. In the latter instance, he recommends a 14-day period.
For her part, Dr. Holekamp said, “I position my folks face down for a week, even although I believe most holes close within three to four days.” She added, “What we practice and what we know scientifically are sometimes slightly different. One of the most amazing things about the ocriplasmin clinical trial is that macular holes closed about one-third of the time without a gas bubble. But with gas tamponade and face-down positioning, they closed 94 percent of the time. It’s hard to change a winning game.” |
Looking Ahead
For Dr. Sebag, the future lies in pharmacologic vitreolysis, and the future of pharmacologic vitreolysis lies in prevention. “The big bang will come when we learn to identify the right patients for inducing a pharmacologic PVD that will prevent anomalous PVD. The two diseases that will be most impacted by this preventive approach will be diabetic retinopathy and exudative AMD. The potential for savings in human and economic terms is staggering.”
Dr. Thompson noted that he anticipates improvements to drugs like ocriplasmin, improved drug delivery systems to enhance the effect at the vitreomacular interface, and a better understanding of how to use the drugs. He also hopes to learn more about simply injecting an air or gas bubble to lyse a very small VMA. “Sometimes the injected air/gas is sufficient to disrupt the adhesion. There’s no real downside except for a transient elevation in intraocular pressure,” he said. In terms of surgery, the main focus will be improvements in safety and postop recovery time. Along those lines, 27-gauge instruments will be available shortly, he said.
Dr. Holekamp hopes that the vitreous itself will get more attention in the future. “We’re doing a lot of research on trying to create a PVD with an injection, but maybe we should be doing research on trying to prevent the vitreous from liquefying in the first place,” she said. After all, vitreous liquefaction causes nuclear cataract, 15 to 20 percent of open-angle glaucoma, retinal tears and detachments, bleeding in diabetics, VMT, macular puckers, and macular holes. “If we could figure out how to prevent vitreous liquefaction, we wouldn’t have these problems. To me, it’s a final frontier of ophthalmic research.”
__________________________
1 Sebag J. Graefes Arch Clin Exp Ophthalmol. 2004;242(8):690-698.
2 Sebag J. Retina. 2009;29(7):871-874.
3 Sebag J et al. Trans Am Ophthalmol Soc. 2009;107:35-44.
4 Wang MY et al. Retina. 2009;29(5):644-650.
5 Jackson TL et al. Retina. 2013;33(8):1503-1511.
6 Duker JS et al. Ophthalmology. 2013;120(12):2611-2619.
7 Pichi F et al. Int Ophthalmol. 2013 Jul 18. [Epub ahead of print.]
8 Hasegawa Y et al. Graefes Arch Clin Exp Ophthalmol. 2009;247(11):1455-1459.
__________________________
Meet the Experts
NANCY M. HOLEKAMP, MD, is director of retina services at the Pepose Vision Institute and clinical professor of ophthalmology and visual sciences at Washington University School of Medicine in St. Louis, Mo. Financial disclosure: Is a consultant to Alimera Sciences, Allergan, Genentech, Regeneron, and Sequenom; is a speaker for Genentech and Regeneron; receives research funds from Allergan and Notal Vision; and has equity in Katalyst.
ANDREAS K. LAUER, MD, is chief of the vitreoretinal division, vice-chair for education, director of the ophthalmology residency program, and associate professor of ophthalmology at Oregon Health & Science University in Portland. Financial disclosure: Receives research grant support from Allergan, the National Institutes of Health, and Oxford Biomedica.
JERRY SEBAG, MD, FACS, FRCOPHTH, FARVO, is professor of clinical ophthalmology at the Doheny Eye Institute in Los Angeles and founding director of the VMR Institute in Huntington Beach, Calif. Financial disclosure: Is a consultant to Alcon and ThromboGenics.
JOHN T. THOMPSON, MD, is assistant professor of ophthalmology at the Wilmer Eye Institute and clinical associate professor of ophthalmology at the University of Maryland in Baltimore. He is also the current president of the American Society of Retina Specialists and cofounder of Retina Specialists, which is a private practice in the greater Baltimore area. Financial disclosure: Is a consultant to Genentech and receives grant support from Genentech and Regeneron. |