By Sanjay G. Asrani, MD, With Paul J. Foster, FRCS, Paul F. Palmberg, MD, PhD, and Robert Ritch, MD, FACS
Download PDF
This article is part of an occasional series of MD Roundtables, in which a group of experts discuss a topic of interest in their field. This month, Sanjay G. Asrani, MD, of the Duke Eye Center, leads a roundtable on decision making for peripheral iridotomies in patients with narrow angles. He is joined by Paul J. Foster, FRCS, of University College London, Paul F. Palmberg, MD, PhD, of Bascom Palmer Eye Institute, and Robert Ritch, MD, FACS, of the New York Eye and Ear Infirmary. Following are edited excerpts from their conversation.
|
Telltale Pigment
|
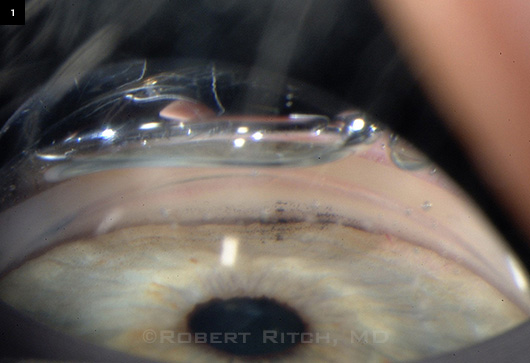 |
Spotty pigment in this narrow angle suggests intermittent angle closure.
|
Which Patients Benefit?
Dr. Asrani: Most of us have been taught that the reason we do iridotomy is to prevent acute angle-closure attacks. Do you feel that prophylactic iridotomy plays a role in preventing other forms of glaucoma, such as chronic angle-closure glaucoma or intermittent angle-closure glaucoma? And if we do a prophylactic iridotomy, will we sometimes limit intermittent angle closure and therefore future glaucoma?
Dr. Ritch: Most patients with narrow angles don’t go on to an acute attack; they’re more likely to get chronic angle closure. If the meshwork is totally clean when you indent [on gonioscopy], that mitigates against intermittent or subacute angle closure. But if you see blotchy pigment on the meshwork (Fig. 1), that suggests the angle is closing in that area, and you are more likely to develop PAS [peripheral anterior synechiae] and chronic angle closure.
So I think of iridotomy as preventing any kind of angle closure, whether acute or chronic, but I think preventing chronic is much more common.
What Does the Evidence Say? Dr. Foster: I think that if one looks at the published evidence, there is not too much yet that would support the performance of iridotomy as a treatment. But what we do know is that if you do an iridotomy, 75 percent of people will end up with a wider-open angle than they had before, and about 25 percent don’t seem to have had much benefit. The evidence we have would suggest that iridotomy probably is a benefit [for patients who have had acute closure] in preventing an acute attack in the unaffected fellow eye.
Evidence for anything else is a bit sketchy at the moment. There is a large clinical trial going on in China with a cohort of about 12,000 people who were screened to identify narrow angles; and, of that number, about 900 had an iridotomy in one eye and not in the other.1
Follow-up is now at three years in the most recently recruited people, and some participants have more than five years of follow-up. We anticipate doing the analysis in that group to look at the prophylactic effect of iridotomy, probably within the next six months.
Regional/Ethnic Differences. Dr. Asrani: In the Far East, the studies being done are on trying to prevent acute angle closure, whereas in the Western world, Africa, and India—and among Hispanic patients—the more common effect of narrow angles is chronic angle closure rather than acute. I think that’s where the difference of opinion exists.
It would be useful to do another study, here in the United States perhaps, to see if we can prevent chronic angle closure.
Dr. Ritch: Why don’t you look at it just on a practical level? It’s a priori logic that if you do iridotomy and open the angle, they’re not going to get chronic angle closure.
Possible Overtreatment. Dr. Foster: The question is whether the people who have narrow angles are really going to go on to get a problem. That’s the key question to address, and we don’t know the answer. Because if we’re doing prophylactic iridotomies in everybody with a narrow angle, we may be overtreating them.
Dr. Ritch: I don’t think I’m overtreating because I’ve got to see evidence of closure first. If the angle doesn’t close, if it’s a slit angle, I tell the patient they can wait. If it’s touching, then they need an iridotomy. But there are lots of doctors out there who were doing iridotomy in grade 3 angles that obviously didn’t need iridotomy.
Dr. Palmberg: When I was on the committee to write the first Preferred Practice Pattern on angle closure for the American Academy of Ophthalmology, we spent an entire day discussing this topic.
All of us concluded that if we saw appositional closure of a couple of clock hours or more superiorly, that was somebody in whom we didn’t want progressive angle closure taking place. But if there were no symptoms of intermittent pain at night in dark situations, if we didn’t see glaukomflecken, or if we didn’t see any peripheral anterior synechiae, then just a narrow angle wasn’t really an indication.
If somebody has an appositional closure and elevated pressure, higher by several points in that eye than the other—and then after the iridotomy, the angle opens and the pressure normalizes over the course of the next month or so, you can look back and say that was something good. Those cases are easy because you already have signs or symptoms of closure.
The tough one to decide is what to do with just a narrow angle, where a very good gonioscopist has looked at it more than a few times.
Patient Factors. Dr. Palmberg: The management of angle closure depends on the patient as well as the findings to some extent. If you have a narrow-angle patient with Alzheimer’s who lives in nursing home and won’t be able to tell you anything, or if you have someone who’s going off to a remote part of the world where treatment isn’t available, a prophylactic laser iridotomy may be a good thing, even if the number needed to treat might be 20 or 50 to 1—if that’s what comes out of Paul Foster’s study.
But if somebody is living in an area with easily available medical care, you’ve explained the symptoms of angle closure, and they’re aware enough—then you can probably just tell them to come in if they have a problem, or else return in six months so you can take a look. And the majority of those people have done fine.
The Problem of Waiting. Dr. Palmberg: But if there’s actually some angle closure, I would argue that you’re starting to see a problem and that you should do something about it.
Looking back historically, in St. Louis, they would do an iridectomy as soon as they saw angle closure, whereas in New York—prior to Bob Ritch—they would keep people on chronic pilocarpine.
But it turned out that even after you did the iridectomy and the angle opened completely, many of the patients in New York (40-50 percent was reported by Max Forbes and others) still needed to be on medication because their trabecular function, even without synechiae, had been compromised over time.
In St. Louis, where the patients with appositional closure and elevated pressure had an iridectomy instead of being put on chronic pilocarpine, the meshwork recovered its function. That was because it hadn’t been deprived of its nutrition, I suppose, by having appositional closure.
So, I think that [outcome] argues in favor of not letting appositional angle closure persist because it could have a permanent effect on the trabecular meshwork function. Sanjay, I think you brought up that point in an article.
Angle Configuration Matters. Dr. Ritch: You also have to think about the configuration of the iris—for example, a patient has a very narrow angle, slit, grade 1. But they have an approach that’s what you might call grade 3, with the height of the iris at the level of the mid-meshwork, so you don’t have to worry so much. They’re not going to have an attack, and they’re not going to get chronic angle-closure glaucoma if you examine them periodically.
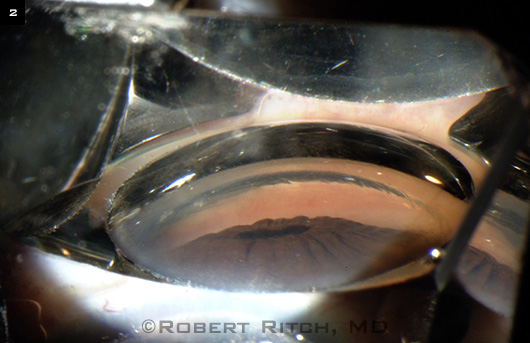
The people you have to be most worried about are those with large lenses, what looks like a partially lensrelated angle closure … the ones with the triple whammy: They have some pupillary block, they have a doublehump sign indicative of some degree of plateau iris, and they have a Mt. Fuji sign (Fig. 2) indicative of some lens component, and the peripheral iris is narrowing up to the level of Schwalbe’s line.
Role of Cataract Surgery. Dr. Palmberg: In recent years, I have been doing far fewer laser iridotomies in cases that were referred to me. Because with just a narrow angle, probably no more than 5 percent go on to get clinical disease before they get their lens snatched out, with cataract surgery being so effective and done at fairly early stages now. So I think that is an important aspect.
Size of Iridotomy
Dr. Asrani: Does the size of the iridotomy matter?
Size Calculations. Dr. Foster: The size definitely does. I see, on a weekly basis, people who have a reasonably sized iridotomy in one eye and a small iridotomy in the other eye. The angles will look manifestly different, but when you enlarge the small iridotomy, the angles then look symmetrical.
We’ve looked at the fluid dynamic calculations again. The earlier calculations done by Dr. Brian Fleck in Edinburgh suggested that at least 50 µm was an acceptable size. We think that is probably an underestimate. We now calculate it to be 200 µm. And I, clinically, feel that’s a bit on the small side, so I would say in excess of 200 µm—as large as you can reasonably make it, given the way the patient tolerates the treatment and the ease with which you can enlarge the iridotomy.
Dr. Asrani: I was initially taught that maybe 50 µm is enough, but 250 µm is where I’m ending up nowadays because I keep enlarging the iridotomy until the fluid gushing from the posterior to the anterior part of the iris slows down dramatically. So I’m glad to hear Paul Foster has the same opinion that 250 µm is an adequate size to equalize the pressure in front of and behind the iris.
Dr. Ritch: I do the small ones. I’m certainly not doing 250 µm—more like 50 µm, and maybe even smaller. If the angle opens, I’m happy. But they can also close up with pigment later on, and I may have to reopen them.
Pigment Granules. Dr. Palmberg: After you make an iridotomy and you see a spurt of fluid, you think you’re done. But I found something out accidentally years ago: Even if you’ve put in a pretreatment with argon and then done YAG to go through, you can have pieces of pigment epithelium get in the way. It’s as if they’re on a string. They can swing into the hole later.
I learned this when I had a patient with Parkinsonism. I made the laser iridotomy, saw the spurt of fluid, and thought I was done. But then, because of his moving rhythmically back and forth against my lens, that shuffling effect of pushing in and out caused pigment granules that were attached to the epithelium to swing into the hole. And I could see it happening.
So I find it helpful to simply push in and out on the gonioprism after making the iridotomy to see if pigment granules come into the hole. If they do, then you just shoot a few more times until that doesn’t happen. I call it the shuffle maneuver. It’s like swishing your mouth after brushing your teeth.
I think that these pigment granules swinging into the hole is one of the causes of finding out that your iridotomy is “inadequately large.” But, of course, if the angle doesn’t open up and get rid of all of the bombé, then whatever you’ve done is not the answer, and Paul Foster is absolutely right—you just keep making it bigger.
Dr. Ritch: There’s a similar phenomenon I used to call “pigment meteors.” You can have pigment particles floating around in the posterior chamber after the iridotomy, and if you have a small opening, it can get blocked. I always look at the patient after half an hour, and if it’s blocked I open it up right away.
 |
|
FUJI SIGN. This “volcano” shape indicateslens involvement in the angle.
|
Considerations for Location
Dr. Foster: In the U.K., we tend to put iridotomies underneath the upper lid and try to avoid them being under the marginal tear strip. So if the patient has a high upper lid margin, we would then consider putting them at, say, either 3 or 9 o’clock position.
Visual Symptoms. Dr. Ritch: Did you notice any difference in symptomatology between doing them at 3 or 9 o’clock versus 12 o’clock?
Dr. Foster: We published a paper in Ophthalmology, where we looked at the size and location of iridotomies, and there isn’t a clear relationship between the symptoms and objective signs of glare and either the location or the size of the iridotomy.2
Line Across the Vision. Dr. Ritch: With the 12 o’clock position, I found that some patients would get a horizontal white line—whether it was from the lid bisecting the iridotomy, which was one of the considerations, or light bouncing off the tear film in the inferior lid and then going up and hitting the upper lid and getting into the eye, which was another concept.
Dr. Palmberg: There clearly are cases where the iridotomy is at the location of the upper tear film, and it makes sense optically that it would give you a line across the vision because you have a cylinder of fluid there. I think that Doug Anderson might have been the first to point this out.
You can check this by putting a patient in a dark room with a light at the other end. They see this line across their vision, and when you have them open their eye a little more or close it more so that the tear film is not over that hole, the line disappears.
There could be other complaints of glare that may or may not be related, but that specific optical phenomenon is so clearly related to a tear film over the hole that—whatever you do—you do not want to have the tear film over that hole because it can make some people miserable.
Dr. Ritch: You can tell people, “Okay, the horizontal white line is due to the iridotomy. Your brain will get used to it.” They are worried more than anything else, and if they stop worrying, most of them don’t get any more symptoms.
But for those who really complain about symptoms, and the iridotomy is at 12 o’clock, it’s possible to put in a couple of contraction burns on the iris peripheral to the iridotomy and pull the position of the iridotomy more peripherally so that it’s completely covered by the lid.
Dr. Asrani: That’s a good suggestion. This optical phenomenon was happening in about 5 percent of all of my iridotomies, and it was happening in the younger people in whom the peripheral lenses were clear. So, in 2009, I changed the location of all my iridotomies to nasal. And since then, the incidence has gone down to like 0.1 percent—so it is currently one in a thousand.
___________________________
1 Jiang Y et al. Ophthalmic Epidemiol. 2010; 17(5):321-332.
2 Congdon N et al. Ophthalmology. 2012; 119(7):1375-1382.
___________________________
Sanjay G. Asrani, MD, is professor of ophthalmology at Duke Eye Center, Durham, N.C. Financial disclosure: Is a lecturer for Alcon, Heidelberg, and Lumenis.
Paul J. Foster, FRCS, is professor at University College London Institute of Ophthalmology and at Moorfields Eye Hospital, London. Financial disclosure: Is a consultant for Alcon and receives research support from Heidelberg.
Paul F. Palmberg, MD, PhD, is professor of ophthalmology at Bascom Palmer Eye Institute, Miami. Financial disclosure: Is an advisor to Allergan, Merck, and Pfizer.
Robert Ritch, MD, FACS, is the Shelley and Steven Einhorn Distinguished Chair, professor of ophthalmology, Chief of Glaucoma Services, and Surgeon Director Emeritus, New York Eye and Ear Infirmary, New York, N.Y. Financial disclosure: Is a consultant for iSonic and Sensimed and has a patent interest in Ocular Instruments.