By Lori Baker-Schena, MBA, EdD, interviewing M. Tariq Bhatti, MD, Frederick W. Fraunfelder, MD, MBA, and April K.S. Salama, MD
Download PDF
When medical oncologist April K.S. Salama, MD, launched her practice in 2010, her patients with malignant melanoma had 2 treatment options. And while both drugs were FDA-approved, neither had demonstrated an overall survival benefit. In most instances, the best she could offer her patients with advanced melanoma was supportive care and hospice.
One short year later, the outlook for many of her patients changed dramatically with the introduction of ipilimumab, the first FDA-approved entry in a class of immunotherapy drugs known as immune checkpoint inhibitors. “This was the first agent that had ever demonstrated a survival benefit in malignant melanoma patients, some of whom are now long-term survivors,” said Dr. Salama, at Duke University in Durham, North Carolina.
New Benefits, New Risks
As Dr. Salama put it, “Recent advances in genetics and immunotherapy have led to more drugs and drug combination approaches, revolutionizing the treatment landscape.”
Yet these advances are not without their risks, including ocular toxicity linked to the drugs’ mechanism of action. These risks caught the attention of M. Tariq Bhatti, MD, also at Duke,1 and Drs. Bhatti and Salama subsequently conducted an extensive review on the neuro-ophthalmic side effects of molecularly targeted cancer drugs.2
“Despite the notion that increased tumor cell specificity results in decreased complications, toxicity remains a major hurdle in the development and implementation of many of the targeted anticancer drugs,” Dr. Bhatti said. “While ophthalmologists do not necessarily need to memorize the nuances of each of these new cancer drugs, they do need to be aware of the recent advancements and their possible effect on their patients.”
 |
|
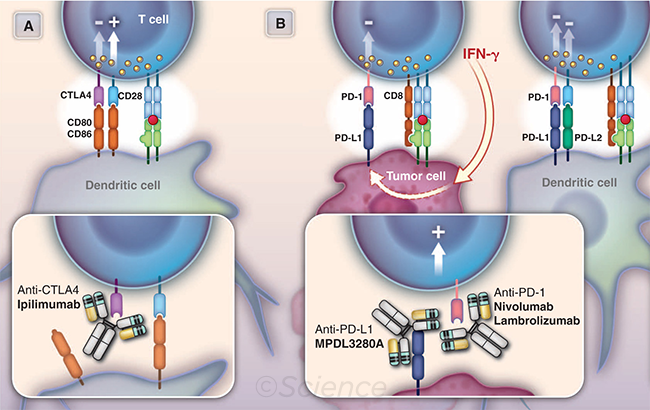
ANTIBODY TX. This illustration shows the use of antibodies in active and passive immunotherapy of cancer. (A) Checkpoint blockade by anti-CTLA4. CD80 and CD86 bind to the costimulatory molecule CD28 to help activate T-cell proliferation and then to the checkpoint inhibitor CTLA4 to attenuate T-cell proliferation. The antibody ipilimumab blocks the interaction of CTLA4 with its ligands, thereby releasing the checkpoint inhibitor and favoring T-cell proliferation. (B) Checkpoint blockade and inhibiting immune suppression by anti–PD-1 or anti–PD-L1. Generally, inhibition of both PD-L1 and PD-L2 (e.g., by anti–PD-1) is required to block negative regulation by dendritic cells, whereas only PD-L1 inhibition (by anti–PD-1 or anti–PD-L1) should relieve immunosuppression (immune rheostat) activity in the tumor bed. Note that, for clarity, only the primary interactions of PD-1, PD-L1, and PD-L2 are illustrated. Reprinted with permission from Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341(6151):1192-1198.
|
A Developing Story
Molecularly targeted therapies can be grouped into 4 broad categories: monoclonal antibodies (mAbs), immune checkpoint inhibitors, small molecule kinase inhibitors, and third-generation aromatase inhibitors.
Want to hear more from these experts?
Attend “Don’t Tell Me You’re Seeing Double!?” at AAO 2017!
Register now
While these drugs hold great promise in terms of efficacy and tolerability, many potentially toxic side effects, including those related to the eye, remain unknown. “Many of the molecularly targeted cancer drugs have unique adverse effect (AE) profiles based on their underlying mechanism of action,” Dr. Bhatti said.
Unanticipated downstream effects. As science pushes the envelope of immunotherapy and immunomodulation, “we are opening ourselves to the unknown,” Dr. Bhatti noted. “Even if [a researcher has] a specific molecular target in mind, we often do not have a true appreciation or understanding of the downstream effects.”
The PML example. He referenced the mAb natalizumab, which demonstrated great efficacy when used to treat multiple sclerosis. “But what was completely unanticipated was the development of progressive multifocal leukoencephalopathy (PML), a rare infectious disorder of the central nervous system that occurs secondary to the John Cunningham virus,” Dr. Bhatti said.
Natalizumab was approved by the FDA in 2004 and withdrawn from the market a year later after 3 cases of PML were identified in clinical trials. It was reintroduced to the market in 2006, with subsequent reports of PML in patients taking the drug. “No one could have predicted this AE,” Dr. Bhatti said. (Natalizumab isn’t the only mAb to be associated with the risk of PML; others include alemtuzumab, bevacizumab, brentuximab vedotin, cetuximab, ibritumomab tiuxetan, and rituximab.)
Missing puzzle pieces. The reporting of AEs can be “inconsistent and difficult to interpret,”3 Dr. Bhatti cautioned, and information can be missing from package inserts4 once a drug is on the market.
A Look at the Timeline
In the past 2 decades, improved understanding of the cellular, molecular, and genetic processes involved in human pathology has led to advances in molecularly targeted therapy.
In this more personalized treatment approach in the fight against cancer, specific cellular molecules (overexpressed, mutationally activated, or selectively expressed proteins) are manipulated to decrease the transformation, proliferation, and/or survival of selected cells, Dr. Bhatti said.
“The FDA approval of rituximab ushered in a new era of targeted therapy for cancer,” said Dr. Bhatti. “And the approval of ipilimumab in 2011 was the first of several next-generation drugs that signaled a resurgence in immunotherapy options for cancer.”
|
Selected Toxicity Profiles
While molecularly targeted drugs are too numerous to mention in this article, the following overview provides a few examples of ocular toxicityassociated with this new generation of anticancer drugs.
Monoclonal antibodies. Currently, 14 FDA-approved mAbs are available. Their mechanism of action includes apoptosis, activation or inhibition of a surface cell receptor, antibody-dependent cellular cytotoxicity, and complement-dependent cytotoxicity. Additionally, mAbs can target tumor cells, immune cells, or vascular/stromal cells.
“In general terms, the ocular toxicity profile of mAbs is good,” Dr. Bhatti said.
Example: Ado-trastuzumab emtansine. This drug has been reported to cause dry eye, blurred vision, cataract formation, conjunctivitis, photophobia, and lacrimal duct edema.
Immune checkpoint inhibitors. These drugs activate the immune system by blocking the immune inhibitory pathways activated by cancer cells. Four drugs in this class are on the market.
“Immune checkpoint inhibitors have a unique safety profile because the immune system is activated, resulting in immune-related AEs, which are common,” Dr. Bhatti said. This subset of AEs occurs in 70% to 90% of patients and can affect multiple organ systems, he said. Fortunately, the incidence of ocular AEs associated with these drugs is significantly less—approximately 1%.
Example: Ipilimumab. Ocular AEs noted with this drug include blepharitis, choroidal neovascularization, conjunctivitis, keratitis, episcleritis, scleritis, and uveitis. In addition, there have been reports of neuroretinitis, myasthenia gravis, and optic neuritis.
Small molecule kinase inhibitors. These drugs affect the intracellular signal pathways that are dysfunctional in cancer cells. FDA approval of the first small molecule kinase inhibitor, imatinib, occurred 15 years ago; as of 2016, 30 of these drugs were on the market.
Example: Crizotinib. In 2 open-label, randomized trials reported in the package insert for crizotinib, 60%-70% of patients experienced a “vision disorder” further defined as blurred vision, diplopia, photophobia, photopsia, reduced visual acuity, visual impairment, and vitreous floaters.
Third-generation aromatase inhibitors. Three FDA-approved third-generation aromatase inhibitors (AIs) are used to treat breast cancer in postmenopausal patients. Unlike the breast cancer drug tamoxifen—which blocks estrogen from binding to the estrogen receptor—the third-generation AIs inactivate the cytochrome P450 enzyme aromatase, thereby reducing production of estrogen from androgens. (Note: Tamoxifen is known to cause ocular side effects, including cataracts, macular edema, and retinal deposits.)
Example: Anastrozole. One study documented an 11.4% frequency of retinal hemorrhage in patients taking anastrozole.5 All affected patients had a single hemorrhage. “The authors hypothesized that a decrease in estrogen level either compromised the vascular system or resulted in vitreoretinal traction,” Dr. Bhatti noted.
Keeping Current
As more cancer drugs are introduced, ophthalmologists will be challenged to keep up with their potential ocular AEs.
National registry. Stay informed through the National Registry of Drug-Induced Ocular Side Effects, founded in 1976 and supported by the Academy and the Casey Eye Institute at Oregon Health & Science University in Portland, Oregon.
Frederick W. Fraunfelder, MD, MBA, at the University of Missouri School of Medicine in Columbia, Missouri, and director of the Registry, noted that while the introduction of drugs in some classes (such as antibiotics) has slowed down, anticancer drugs have “really exploded” onto the market. And given that they have different mechanisms of action that can affect the eyes, “there are some hidden side effects we are just starting to uncover,” he said.
Published studies. “It is incumbent upon ophthalmologists to stay current with high-impact literature, which includes the journals in our profession that affect our patients,” Dr. Fraunfelder said. “It is impossible to know every ocular AE of every drug, but we need to stay current on the big issues.”
Implications for Practice
Dr. Salama noted that she has seen patients with uveitis in her medical oncology practice.
Collaboration. “It is important that ophthalmologists work closely with a patient’s oncologist should ocular side effects arise,” Dr. Salama said. In addition, she said, ophthalmologists should be aware that the long-term prognosis for many patients has dramatically improved: “While some of these patients once had weeks or months to survive, many are now long-term survivors, and proper and timely management of these ocular side effects contributes greatly to the quality of their lives.”
Dr. Bhatti agreed, and he cited an issue that needs to be handled with great sensitivity: “These patients often have all their hopes tied to their medication,” he said. “Therefore, stopping treatment is a difficult decision, and it cannot be made without the input of the patient and the oncologist.” Fortunately, he added, “in some cases, the drug can be successfully continued if a specific management plan is instituted.”
Detailed history. Dr. Bhatti also suggested conducting a detailed history of patients to discover whether they are taking one of the molecularly targeted cancer therapies. “As ophthalmologists, we do need to be aware that the field of cancer treatment has advanced significantly,” he said.
___________________________
1 Bhatti MT. Neuro-ophthalmic side effects of new cancer drugs. Presented at: 43rd Annual Meeting of the North American Neuro-Ophthalmology Society; April 6, 2017; Washington, D.C.
2 Bhatti MT, Salama AKS. Eye (Lond). 2017; in press.
3 Scharf O, Colevas AD. J Clin Oncol. 2006;24(24):3933-3938.
4 Seruga B et al. J Clin Oncol. 2011;29(2):174-185.
5 Eisner A et al. Optom Vis Sci. 2008;85(5):301-308.
___________________________
Dr. Bhatti is chief of the neuro-ophthalmology division and professor in the departments of ophthalmology, neurology, and neurosurgery at Duke Eye Center and Duke University Medical Center in Durham, N.C. Relevant financial disclosures: Novartis: L; Receptos: C.
Dr. Fraunfelder is chairman and Roy E. Mason and Elizabeth Patee Mason Distinguished Professor of Ophthalmology at the University of Missouri School of Medicine in Columbia, Mo. Relevant financial disclosures: None.
Dr. Salama is assistant professor of medicine and a member of the Duke Cancer Institute at Duke University Medical Center in Durham, N.C. Relevant financial disclosures: Bristol-Meyers Squibb: C,L,S; Genentech: C,S; Merck: S. Note: All grant support paid to Dr. Salama’s institution.
For full disclosures and the disclosure key, see below.