By Jared D. Peterson, MD, Timothy V. Johnson, MD, and Alan R. Forman, MD, FACS
Edited by Steven J. Gedde, MD
Download PDF
Other than needing glasses for high myopia, 55-year-old Mike Miller* had never had any eye problems before. That is why he was so alarmed when he awoke one morning with a red, painful eye. He tried to recall anything out of the ordinary that might have contributed to the condition. In the end, the only thing he could link it to was a sneeze the day before.
Initially, Mr. Miller saw his internist and optometrist for care. To them, he described experiencing a complete whiteout of vision in his right eye, which began just after he held in a sneeze. Mr. Miller’s wife added that he had always been a very loud, violent sneezer. His vision returned to baseline during the 30 minutes after the sneeze and remained normal through the remainder of that day. But the next morning, his eye was red and painful.
Mr. Miller’s optometrist treated him with antibiotic and steroid drops, and his internist increased his dosage of aspirin. However, he experienced no relief. After five days of persistent symptoms, he came to our hospital.
We Get a Look
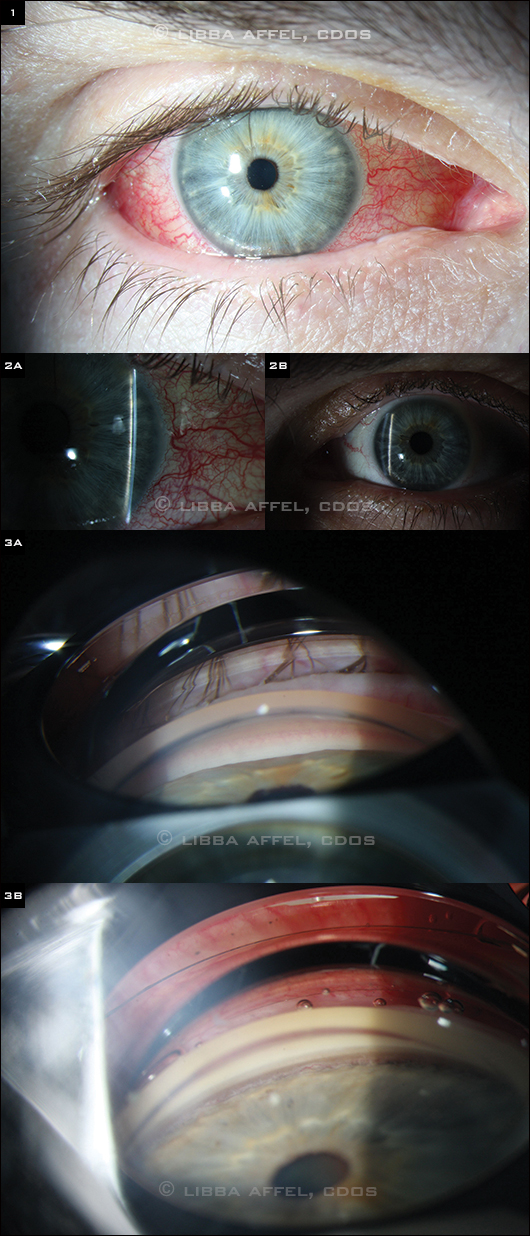
When we examined him, Mr. Miller’s vision was 20/50 and 20/20 in his right and left eyes, respectively. His extraocular motility was full, and his pupils reacted briskly bilaterally with no afferent pupillary defect. His right conjunctiva was diffusely injected with vascular tortuosity and mild chemosis (Fig. 1).
On slit-lamp examination, the corneas were clear and the anterior chambers were quiet in both eyes, but the anterior chamber of the right eye (Fig. 2A) was markedly more shallow than that of the left (Fig. 2B). Mr. Miller had symmetric mild nuclear sclerotic cataracts, and his intraocular pressures were 20 mmHg in his right eye and 10 mmHg in his left. No angle structures were visible in the right eye on gonioscopy (Fig. 3A), while all structures were visible in the left (Fig. 3B). Mr. Miller’s blood pressure was 124/76.
|
What's Your Diagnosis?
|
 |
|
(1) The conjunctiva of the right eye was diffusely injected with vascular tortuosity and mild chemosis. (2A,B) The anterior chamber of the right eye was markedly more shallow than that of the left. No angle structures were visible on gonioscopy in the right eye, while all structures were visible in the left (3A,B).
|
Early Misdiagnoses
Conjunctivitis. Mr. Miller’s optometrist treated him for presumed conjunctivitis with a topical antibiotic and steroid. In this case, however, the abrupt onset of symptoms, transient vision loss, and asymmetric IOPs and anterior chamber depths rendered this diagnosis unlikely.
Amaurosis fugax. Mr. Miller’s internist instructed him to increase his daily aspirin dose, thinking of possible amaurosis. However, amaurosis is characteristically a painless graying or blackening of vision, not a whiteout. The associated pain and abnormal ocular findings were also not consistent with this diagnosis.
Differential Diagnosis
At this point, our differential focused on the following conditions.
Arteriovenous fistula. A vascular fistula must be considered in patients with unilateral pain, injection, vessel tortuosity, chemosis, and a narrow angle.
Primary angle-closure glaucoma (PACG). We considered this diagnosis, given the acute-onset red eye, decreased vision, asymmetric IOPs, and narrow angle. However, the presence of an open angle in the fellow eye of a high myope decreases the likelihood of PACG. Additionally, the axial shallowing of the anterior chamber was not consistent with PACG; rather, it suggested that the lens-iris diaphragm was shifted forward as a result of posterior segment pathology.
Secondary angle closure from a posterior pushing mechanism. Several ocular conditions can produce forward displacement of the lens-iris interface with secondary closure of the anterior chamber angle. Peripheral and central shallowing of the anterior chamber is present, as was seen in our patient. In addition to angle-closure symptoms, these patients often have a significant myopic shift.
- Medications. Certain medications, including topiramate (Topamax) and other sulfa drugs, can cause an acute ciliochoroidal effusion, usually bilaterally, displacing the lens-iris complex forward into angle closure.
- Tumors. A choroidal or retinal tumor can shift the lens and iris forward as the tumor enlarges, depending on its size and location. Choroidal melanoma, retinoblastoma, and ocular metastases are the tumors that most commonly cause secondary angle-closure glaucoma.
- Aqueous misdirection. Also known as malignant glaucoma, aqueous misdirection is most commonly seen after filtering surgery. It also can occur following cataract surgery or laser procedures, and it has occurred spontaneously in rare instances. In this entity, aqueous is misdirected posteriorly, with impaired flow at the level of the lens equator, anterior vitreous face, and ciliary processes. Both the peripheral and central anterior chambers become flattened, and the lens and vitreous face may be pushed forward.
- Uveal effusion and nonrhegmatogenous retinal detachment. Accumulation of fluid or blood in the suprachoroidal or subretinal space can displace the lens-iris diaphragm forward, with associated angle closure.
- Retinal surgery and retinal vascular disease. Scleral buckling procedures, panretinal photocoagulation, and central retinal vein occlusion can produce congestion of the ciliary body, often accompanied by choridal effusion, with anterior rotation and angle closure. Injection of air, expansile gas, or silicone oil with pars plana vitrectomy may also result in secondary angle-closure glaucoma.
Pinning It Down
Many of the above mechanisms for secondary angle closure were excluded in our patient, given the lack of pertinent history. Mr. Miller had not started any new medications, and he had never had eye surgery. A CT angiography performed in the emergency room revealed no sign of an arteriovenous fistula.
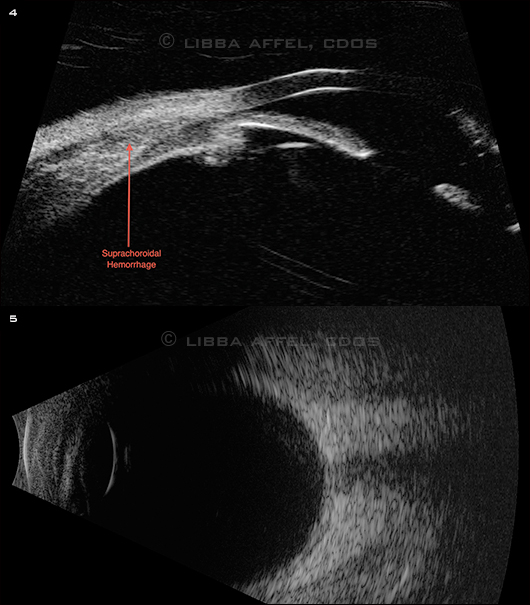
The funduscopic exam of the left eye was normal. The right eye had anterior hemorrhagic choroidals seen without scleral depression through a widely dilated pupil. The anterior suprachoridal hemorrhage was confirmed with UBM (Fig. 4) and B-scan ultrasongraphy (Fig. 5).
 |
|
PROBLEM REVEALED. (4) UBM showed an anterior suprachoroidal hemorrhage. (5) B-scan confirmed that the choroidals were isolated to an anterior location.
|
Patient Update
As Mr. Miller’s choroidals were relatively small and his IOP was not elevated, the decision was made to observe him. Although he was not taking any prescription anticoagulation or anti-platelet agents, he had been taking aspirin without a medical indication. We instructed him to discontinue taking the aspirin. In addition, we instructed him to not stifle his sneezes, with the goal of minimizing any Valsalva effect, which could have initiated the suprachoroidal hemorrhage. One week later, Mr. Miller’s symptoms had completely resolved and his choroidals were much smaller in size, with deepening of the anterior chamber.
Relatively Rare
Suprachoroidal hemorrhage (SCH) is almost always seen in the setting of surgery or trauma. However, spontaneous cases of SCH have been reported. Based on case reports in the literature, risk factors for SCH include advanced age, arteriolar sclerosis, systemic anticoagulation or antiplatelet therapy, and AMD.1-6
The most common symptoms of SCH include pain, nausea, and vomiting; in addition, IOP is often elevated. In situations such as Mr. Miller’s (in which the choroidals are small, pain is mild, and IOP is not overtly elevated), observation is recommended. Because blood in the suprachoroidal space tends to clot quickly, surgical drainage is typically delayed for at least one to two weeks to allow time for the blood to liquefy. One small case series showed that patients who require surgical drainage typically have very poor visual outcomes.6
___________________________
* Patient’s name is fictitious.
___________________________
1 Chen YY et al. J Chin Med Assoc. 2009;72(7):385-387.
2 Nguyen HN, Nork TM. Retina Cases Brief Rep. 2012;6(2):216-218.
3 DeMarco R et al. Eur J Ophthalmol. 2009;19(5):883-886.
4 Tajika T et al. J Med Invest. 2008;55(1-2):151-155.
5 Khawly JA et al. Am J Ophthalmol. 1996;121(5):577-578.
6 Yang SS et al. Retina. 2003;23(2):139-144.
___________________________
Drs. Peterson and Johnson are residents in ophthalmology, and Dr. Forman is a cataract and comprehensive ophthalmologist; all are at Wills Eye Institute in Philadelphia. The authors report no related financial interests.
Calling All Cases!
Submit your mysterious case to:
Morning Rounds EyeNet Magazine655 Beach Street San Francisco, CA 94109 eyenet@aao.org |