By Dilraj S. Grewal, MD, and Robert S. Feder, MD, MBA
Edited by Steven J. Gedde, MD
Download PDF
Brad Smith* was frustrated. He needed new glasses again, for the fourth time in as many years. The 54-year-old insurance executive came to us complaining of progressive blurred vision in his left eye and noted that he had received an increasing myopic correction for that eye several years in a row. During that time, he told us, the vision in his right eye had not changed.
We Get a Look
Mr. Smith reported that he had undergone bilateral upper lid blepharoplasty in his early 40s and uncomplicated cataract surgery in both eyes seven years before we saw him. He also reported a medical history of well-controlled psoriasis and allergic rhinitis.
When we examined him in 2012, Mr. Smith’s manifest refraction in his left eye was –1.50 + 1.00 x 145 (changed from –0.50 + 0.50 x 135 in 2009) and the best-corrected visual acuity (BCVA) in that eye was 20/40 (changed from 20/25 over the same time period). The BCVA in his right eye had held steady at 20/25.
There was no afferent pupillary defect and his visual fields were full to confrontation. The intraocular pressures were 12 mmHg in the right eye and 10 mmHg in the left by applanation.
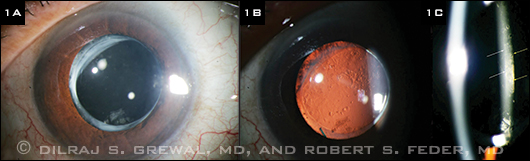
Examination of the right eye showed a normal anterior segment with a well-positioned posterior chamber IOL (PCIOL). The left eye revealed a clear cornea with a normal contour (average keratometry of 44.2 D) and a well-centered PCIOL in the bag (Fig. 1A). Retroillumination revealed the presence of mild posterior capsule opacification (PCO; Fig. 1B).
In using a fine slit beam, we noted that the capsule was distended posteriorly. Moreover, it was evident that a translucent liquid had accumulated between the IOL optic and the posterior capsule (Fig. 1C). The dilated fundus exam of both eyes was unremarkable.
|
What's Your Diagnosis?
|
 |
|
LEFT EYE. (1A) A well-centered posterior chamber IOL was present in the bag of the left eye. (1B) Mild PCO was noted. (1C) The posterior capsule was distended posteriorly, and translucent fluid had accumulated between the IOL optic and the posterior capsule. (White arrows show the posterior edge of the IOL; yellow arrows the anterior edge of the posterior capsule.)
|
Differential Diagnosis
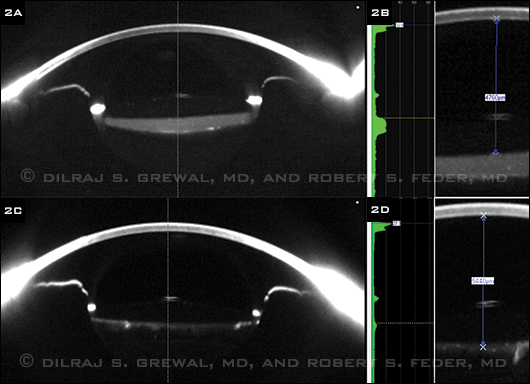
At this point, our differential diagnosis included capsular bag distension syndrome, posterior capsule opacification (PCO) with Elschnig pearls, and the remote possibility of sequestered cortical material or retained viscoelastic. We performed Scheimpflug imaging with a Pentacam (Fig. 2A), which clearly demonstrated a distended, fluid-filled capsular bag. This established the diagnosis of capsular bag distension syndrome.
 |
|
PINNING IT DOWN. (2A) Scheimpflug imaging showed that the capsular bag was distended and filled with fluid. (2B) An Nd:YAG posterior capsulotomy resulted in the loss of fluid from the capsular bag, resolution of the distension, and a posterior shift of the IOL optic. (2C) Before the capsulotomy, the distance from the corneal endothelium to the anterior optic surface measured 4,760 µm. (2D) This distance increased to 5,440 µm following the procedure, thus confirming a 680 µm posterior displacement of the IOL optic.
|
Management
We performed an Nd:YAG posterior capsulotomy. Following this procedure, there was posterior egress of fluid from the capsular bag into the vitreous and resolution of the distension seen on the preoperative Scheimpflug images (Fig. 2C).
The measurement calipers on the Pentacam software allowed us to calculate the dimensions of the distended capsular bag. Prior to the capsulotomy, the distance from the corneal endothelium to the anterior optic surface was 4,760 µm (Fig. 2B). Following the capsulotomy, this increased to 5,440 µm (Fig. 2D), demonstrating that the IOL optic moved 680 µm posteriorly as a result of the evacuation of the capsular bag.
Using the Pentacam, we were able to estimate the turbidity of the material filling the capsular bag. It measured 6.4 units on the densitometry scale, compared with 2 units for the density of the IOL and 1.5 units for the aqueous. Using the software, we also were able to create three-dimensional reconstructed tomograms, which also demonstrated the resolution of the capsular bag distension. Following the capsulotomy, the manifest refraction in the left eye significantly improved to –0.25 + 0.50 x 145, and the BCVA in that eye improved to 20/25.
Discussion
Capsular bag distension syndrome—also known as viscoelastic entrapment syndrome, capsular bag hyperdistension, capsulorrhexis block syndrome, and capsular bag syndrome—is an uncommon and rarely recognized finding that occurs after phacoemulsification involving continuous curvilinear capsulorrhexis and IOL implantation.
First described by Davison in 1990,1 the syndrome occurs when a liquefied substance accumulates in the capsular bag following phacoemulsification when the capsulotomy is occluded by the anterior surface of the IOL. The most dramatic feature of the syndrome is posterior distension of the posterior capsule into the anterior vitreous cavity. However, progressive postoperative myopia and a shallowing of the anterior chamber caused by an anterior shift of the IOL optic are also characteristic.
Overview
Classification. Miyake et al. established a classification system that differentiates the intraoperative, early postoperative (occurring one day to two weeks postoperatively), and late postoperative (taking place, on average, about 3.8 years postoperatively) syndromes.2 Occurrence during the early postoperative period is most common of the three.
Mechanism. It is thought that early postoperative distension occurs when intracapsular fluid is prevented from escaping the capsular bag by an anteriorly displaced IOL that is adherent to the anterior capsule.
However, the possibility of an osmotic gradient secondary to the presence of sodium hyaluronate in ophthalmic viscoelastic devices (OVDs) in the capsular bag has also been suggested.3 In this scenario, the hyperosmolar OVD causes the aqueous humor to be drawn into the capsular bag to reestablish the osmotic balance. Lending support to this theory, studies analyzing the material accumulated in the capsular bag have found it to contain Propionibacterium acnes (anaerobic gram-positive rod) and sodium hyaluronate.3,4
The mechanism of late capsular bag distension syndrome is thought to be secondary to fibrosis and adhesions between the anterior capsule and the anterior edge of the IOL optic.5
Treatment. Options for treating capsular bag distension syndrome include an anterior or posterior Nd:YAG laser capsulotomy, depending on the clinical scenario.
A Note Regarding Myopic Shift
Scheimpflug imaging helped us estimate the refractive change following capsulotomy. It has been demonstrated that a 1-mm anterior displacement of the IOL correlates to 2 D of induced myopia for a corneal surface with an average curvature of 44 D.6 In our case, the 680-µm anterior displacement would be expected to induce 1.3 D of myopic shift.
This was close to the 1.25 D evident on manifest refraction after the posterior capsulotomy, and it allowed us to determine that most of the induced myopia was accounted for by the anterior displacement of the IOL.
Conclusion
Capsular bag distension syndrome is an uncommon finding that can occur several years after uncomplicated phacoemulsification and implantation of an IOL.
It does not correspond to the degree of PCO; in fact, the posterior capsule may be relatively clear. Clinicians should consider this condition in the differential diagnosis of unexpected postoperative myopia following IOL implantation.
Treatment is simple and effective and consists of an Nd:YAG posterior capsulotomy. Scheimpflug imaging techniques, as illustrated in this report, can help confirm both the diagnosis and the resolution.
___________________________
* Patient’s name is fictitious.
___________________________
1 Davison JA. J Cataract Refract Surg. 1990;16(1):99-108.
2 Miyake K et al. J Cataract Refract Surg. 1998;24(9):1230-1234.
3 Sugiura T et al. J Cataract Refract Surg. 2000;26(3):420-425.
4 Dhaliwal DK et al. Arch Ophthalmol. 2011;129(2):246-247.
5 Theng JT et al. J Cataract Refract Surg. 2000;26(3):462-467.
6 Sorenson AL et al. Ophthalmology. 2000;107(5):902-908.
___________________________
Dr. Grewal is a resident in ophthalmology and Dr. Feder is professor of ophthalmology; both are at Northwestern University in Chicago. This manuscript was supported in part by an unrestricted grant to Northwestern’s Department of Ophthalmology from Research to Prevent Blindness in New York. Drs. Grewal and Feder report no other related financial interests.
Calling All Cases!
Interested in sharing a mysterious or challenging case with your colleagues? Submit it to us at:
Morning Rounds EyeNet Magazine655 Beach Street San Francisco, CA 94109 Phone: 866-561-8558 or 415-561-8500 eyenet@aao.orgPlease contact us regarding writers guidelines and art requirements. Samples of previous columns also can be provided. |