By Jessica L. Chen, MD, and Richard K. Lee, MD, PhD
Edited by Steven J. Gedde, MD
Download PDF
When Cindy Gonzales* visited her optometrist, she anticipated that it would just be a routine examination for eyeglasses. But things took a turn for the worse when the optometrist found that the 31-year-old attorney’s IOP was 28 mmHg in her right eye and 26 mmHg in her left. He promptly referred Ms. Gonzales to our glaucoma clinic for further evaluation.
We Get a Look
Ms. Gonzales reported having a history of intermittent headaches and blurred vision whenever she pursued any vigorous activity, such as running. She was not using eyedrops and had not undergone any laser or surgical treatment, and she denied having any history of ocular trauma. Ms. Gonzales was otherwise healthy and not taking any systemic medications.
When asked about her family history, she noted that her paternal grandmother was blind secondary to glaucoma and that her maternal grandfather and great-aunt both were diagnosed with glaucoma late in life.
Upon examination, Ms. Gonzales’ BCVA was 20/20 in both eyes with a refractive error of –2.50 D sphere in her right eye and –3.00 D sphere in her left. Her IOP was 22 mmHg in the right eye and 23 mmHg in the left. Both pupils were 3 mm, and they were reactive and without an afferent pupillary defect. Ocular movements were full in both eyes. Her confrontation visual fields were full in both eyes. Humphrey visual field testing was normal in both eyes.
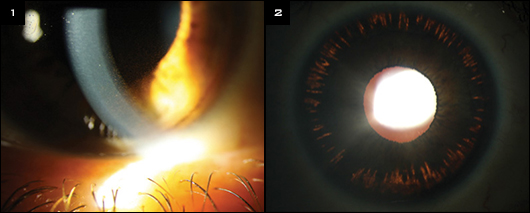
The slit-lamp examination showed clear corneas with prominent Krukenberg spindles (Fig. 1) and a deep anterior chamber in both eyes. Gonioscopy revealed a peculiar iris contour that bowed posteriorly as well as heavy pigmentation of the trabecular meshwork, without any peripheral anterior synechiae. Her central corneal thickness was 565 µm in both eyes, and midperipheral iris transillumination defects in a spokelike pattern were present 360 degrees (Fig. 2). Her lenses were clear.
The dilated fundus exam revealed optic nerves that appeared healthy, with a cup-to-disc ratio of 0.25, intact rims, and no disc hemorrhage or notching. The macula, vessels, and periphery were within normal limits in both eyes.
There was no question in our minds that our patient had pigment dispersion syndrome.
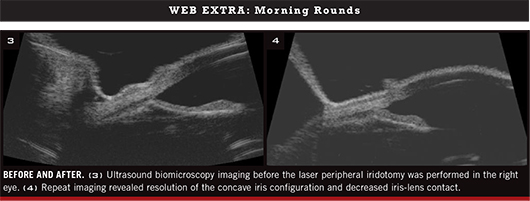
A week later, Ms. Gonzales returned for a follow-up exam. We ordered ultrasound biomicroscopy (UBM) imaging with provocative light testing. Imaging in bright light caused pupillary constriction and demonstrated posterior bowing of the iris, with reverse pupillary block and contact between the posterior surface of the iris and the anterior lens and zonules, as compared with imaging in the dark.
|
What's Your Diagnosis?
|
 |
|
(1) Prominent Krukenberg spindles were evident in both eyes at the slit lamp. (2) In addition, there were peripheral iris transillumination defects in a spokelike pattern in both eyes.
|
Diagnosis and Treatment
Pigment dispersion syndrome (PDS) was first described in 1979 by Campbell, who proposed the mechanism of lens zonular fibers rubbing against the posterior iris surface, thereby causing pigment release that leads to aqueous outflow obstruction.1 The prevalence of PDS in the Caucasian population is estimated to be 2.45 percent.2
The diagnosis. Clinical findings include midperipheral iris transillumination defects; a concave iris configuration; and pigment deposits on the lens, zonules, angle, and cornea.
Preventing conversion. The primary factor that predicts the conversion of PDS to pigmentary glaucoma is elevated IOP at the initial examination: An IOP greater than 21 mmHg increases the risk of conversion by 25-fold.2
In 1992, Karickhoff proposed a possible prophylactic treatment—laser peripheral iridotomy (LPI)—to prevent conversion of PDS to glaucoma.3 He agreed with the mechanism of reverse pupillary block first described by Campbell, in which aqueous can flow into the anterior chamber but is blocked from equilibrating with the posterior chamber because the iris acts like a flap valve against the lens surface. As the aqueous collects in the anterior chamber, the pressure gradient displaces the iris posteriorly, which causes increased iridozonular contact and additional pigment shedding.
Theoretically, an LPI would equalize the pressures between the posterior and anterior chambers, minimize iridozonular contact, and restore the normal iris configuration—and, in doing so, decrease pigment dispersion.2
(click to expand)
LPI and Long-Term IOP Control
Several studies have attempted to determine whether an LPI can reduce the conversion rate of PDS with ocular hypertension to pigmentary glaucoma. However, this question has yet to be definitively answered.
No benefit? For instance, a randomized controlled study found no benefit from Nd:YAG LPI in preventing progression from PDS to pigmentary glaucoma within three years of follow-up. However, the inclusion criterion in this study was ocular hypertension (OHT), defined as IOP greater than 21 mmHg without treatment, and the average age of study participants was approximately 49 years old.2 The researchers note, “It is possible that the onset of OHT in PDS may indicate a combination of pathologic changes that are irreversible.”2
It is plausible that if LPI is performed early enough in the course of the disease, before OHT develops, it may be effective in reducing the risk of developing pigmentary glaucoma. (However, the conversion rate from pigmentary dispersal to pigmentary glaucoma ranges from 10 to 50 percent; thus, an LPI would be unnecessary in many patients.) LPI may have greater benefit for patients who demonstrate reverse pupillary block on UBM imaging than for those patients who do not have this finding.
Selection bias? In a retrospective study, members of the American Glaucoma Society were surveyed to determine whether LPI was associated with a subsequent decrease in IOP in patients with pigmentary dispersion. For 46 of 60 patients with more than two years of follow-up, a greater IOP decrease was observed in the eye treated with LPI, compared with the fellow eye. However, statistical analysis revealed that this difference was likely due to selection bias.4
Back to Our Patient
Given the presence of posterior bowing of the iris and potential involvement of a reverse pupillary block mechanism, we explained the risks and benefits of an LPI (including the lack of evidence supporting prophylactic use) as well as alternative treatments to Ms. Gonzales. She consented to an LPI in one eye, with a plan to consider the procedure in the fellow eye if a beneficial effect was observed. We advised her to avoid strenuous exercise and to immediately come in for evaluation if she experienced eye pain or blurred vision following physical activity.
An LPI was performed in Ms. Gonzales’ right eye. Her IOP was measured one hour after the procedure and was not elevated at that time. One week later, it was normal. One month later, her IOP had decreased to 16 mmHg in both eyes. In addition, repeat UBM imaging with provocative light testing revealed resolution of the concave iris configuration and decreased iris-lens contact in the right eye.
We are continuing to observe Ms. Gonzales’ fellow eye. Given her age and the presence of anatomic findings that are consistent with reverse pupillary block, we hope that this intervention will decrease her risk of progression.
___________________________
* Patient’s name is fictitious.
___________________________
1 Campbell DG. Arch Ophthalmol. 1979;97(9):1667-1672.
2 Scott A et al. Ophthalmology. 2011;118(3):468-473.
3 Karickhoff JR. Ophthalmic Surg. 1992;23(4):269-277.
4 Reistad CE et al. J Glaucoma. 2005;14(4):255-259.
___________________________
Dr. Chen practices with the Palo Alto Eye Group in Palo Alto, Calif. Dr. Lee is associate professor of ophthalmology at the Bascom Palmer Eye Institute. The authors report no related financial interests.