By Ryan Rosenblatt, Jon S. Poling, MD, PhD, and Mohan N. Iyer, MD
Edited by Steven J. Gedde, MD
Download PDF
Ronald Walker* is a 56-year-old schoolteacher who noted a “wiggle” in his vision every time he ate sweets or drank coffee. It happened simultaneously in both eyes after ingestion of the trigger substances and usually resolved after he took ibuprofen. However, with the most recent episode, Mr. Walker experienced the wiggle with no relief for five weeks, despite taking ibuprofen. He went for an eye exam and was diagnosed with an epiretinal membrane and posterior vitreous detachment, and was referred to our office for further evaluation.
We Get a Look
Mr. Walker told us about the ongoing visual disturbance at the 10 o’clock position in both eyes. Visual acuity was 20/25 in the right eye and 20/20 in the left. IOP was 11 mmHg in the right eye and 8 mmHg in the left. No afferent pupillary defect was noted, and ocular motility was full. Visual fields were grossly full to confrontation, but on Amsler grid testing he noted paracentral metamorphopsia at 10 o’clock up and to the left with both eyes. His past medical history was significant for mild obstructive sleep apnea, abnormal cardiac rhythm, and headaches with associated visual disturbance. He told us that the only medication he took was ibuprofen as needed.
His previous episodes of visual disturbance suggested a diagnosis of acephalgic migraine. Clinical exam and OCT ruled out retinal pathology. We obtained a visual field test to further assess the new visual disturbance.
The Visual Field Test
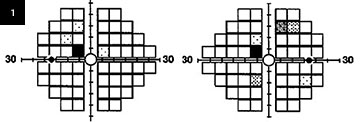
Visual field testing showed a scotoma within 10 degrees of fixation in the left superior quadrant in both eyes (Fig. 1).
Our understanding of visual field defects related to brain damage dates back to the work of Tatsui Inouye and Gordon Holmes, among others, during the early 20th century.1 Dr. Inouye, a Japanese ophthalmologist, and Dr. Holmes, a British neurologist, studied head wounds sustained during the Russo-Japanese War and World War I, respectively. Based on these injuries—and the resultant visual defects—Dr. Holmes created a diagram of the probable representation of different segments of visual field in the calcarine cortex. According to his retinotopic map, the left homonymous paracentral superior quadrantic scotoma would be expected to correspond to a defect at the tip of the right occipital lobe in the inferior cortex of the calcarine fissure.2 Dr. Holmes’ map became the accepted standard for several decades but was noted to have underestimated the magnification of central vision on the calcarine cortex by assigning only 25 percent of the cortex to the central 15 degrees of visual field.1 The map was updated by Horton and Hoyt using magnetic resonance imaging (MRI) to study the effects of recent infarcts in the calcarine cortex on visual fields.3 The now-accepted Horton and Hoyt map depicts the magnification of the central field on the calcarine cortex and assigns the central 24 degrees of visual field to 80 percent of the cortex.3
The work of Horton and Hoyt is more applicable to Mr. Walker, who had not experienced any head trauma or wounds, which suggested the hypothesis that a compressive lesion or infarction was the cause of the wiggle.
|
What's Your Diagnosis?
|
 |
|
24-2 Humphrey visual field testing reveals a left homonymous paracentral superior quadrantic scotoma and a few nonspecific visual field changes.
|
Additional Testing and Treatment
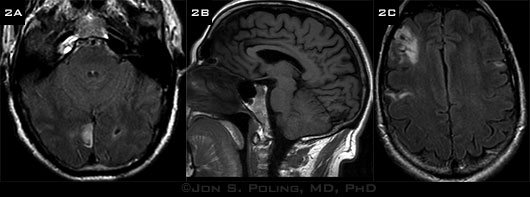
We sent Mr. Walker for a neurologic evaluation and obtained MRI of the brain. His neurologic examination was intact with the exception of the visual field defect. Medical history was negative for major vascular risk factors and yielded only the minor risk factor of obstructive sleep apnea, which was treated with a continuous positive airway pressure device at night. Mr. Walker now told us that for many years he had occasional heart fluttering when he was tired; this symptom dated back to childhood. Neuroimaging not only showed an infarction at the right calcarine cortex as predicted (Figs. 2A and 2B) but also revealed three other areas of chronic encephalomalacia consistent with chronic infarcts bilaterally within the hemispheres (Fig. 2C). MR angiogram did not reveal any significant large vessel stenosis in the head or neck arteries.
Based on the brain MRI results, we strongly suspected a proximal cardioembolic source and sought to confirm our suspicion with additional studies. Three-day cardiac Holter testing showed normal sinus rhythm with occasional premature ventricular contractions and premature atrial contractions. A transthoracic echocardiography with agitated saline bubble study suggested a large patent foramen ovale (PFO) with immediate right-to-left shunting and associated atrial septal aneurysm. This was confirmed by transesophageal echocardiography.
 |
|
MRI. (2A, B) We saw an infarct in the right calcarine cortex and (2C) three chronic infarcts involving both hemispheres.
|
Diagnosis and Discussion
Mr. Walker’s multiple cryptogenic strokes were thought to have occurred due to paradoxical embolism that traveled via the PFO to the brain. Based on autopsy studies, the prevalence of atrial septal defects (ASDs) in the general population ranges from 20 to 26 percent. Patients with ASD or PFO can remain undiagnosed for decades due to lack of significant physical exam findings and symptoms—in fact, most remain asymptomatic, and the role of PFO in cryptogenic stroke remains controversial. The most common symptoms of ASD include exertional dyspnea or fatigue, or patients may develop symptoms related to atrial fibrillation or flutter. Less commonly, symptoms related to paradoxical embolism or transient ischemic attack may be the first manifestation of an atrial septal defect.4 Mr. Walker’s MRI had revealed chronic infarcts, which had been clinically silent.
Management
The appropriate management of ASD in the setting of a cryptogenic stroke remains debatable, with options including medical management, open surgical closure, or transcatheter percutaneous closure with approved devices. The CLOSURE study published in 2012 showed no difference in the two-year stroke rate (of approximately 3 percent) between the medical therapy and percutaneous-closure groups.5 However, the RESPECT trial published in 2013 showed marginal benefit in a secondary analysis of closure utilizing the Amplatzer device.6 Surgical closure requires a sternotomy and cardiopulmonary bypass and now, with improved techniques over the years, has an extremely low mortality rate. Transcatheter percutaneous closure has been reported to have a success rate that compares favorably with surgical closure7 and it requires a shorter hospital stay than does surgery.
Mr. Walker elected to proceed with percutaneous closure of the ASD using an Amplatzer septal occluder in early 2012. Shortly after successful closure of the ASD, Mr. Walker was able to return to his teaching position. He still has the visual disturbance at 10 o’clock, but he reports no new visual or neurological changes in two years of follow-up.
This case illustrates the importance of neuroimaging workup for patients who are thought to have migraine but have atypical features or prolonged “aura” consisting of neurological and visual deficit.
___________________________
* Patient name is fictitious.
___________________________
1 Fishman RS. Doc Ophthalmol. 1997;93(1-2):9-28.
2 Holmes G. Br J Ophthalmol. 1918;2(7):353-384.
3 Horton JC, Hoyt WF. Arch Ophthalmol. 1991;109(6):816-824.
4 Webb G, Gatzoulus MA. Circulation. 2006;114(15):1645-1653.
5 Furlan AJ et al. N Engl J Med. 2012;366(11):991-999.
6 Carroll JD et al. N Engl J Med. 2013;368(12):1092-1100.
7 Kutty S et al. Am J Cardiol. 2012;109(9):1348-1352.
___________________________
Mr. Rosenblatt is a premedical student at the University of Georgia and has worked as an ophthalmic assistant at the Athens Retina Center, Athens, Georgia. Dr. Iyer and Dr. Poling are in private practice at Athens Retina Center and Athens Neurology Associates, respectively. The authors report no related financial interests.