By Ling Y. Bei, MD, and Collin M. Mcclelland, MD
Edited by Steven J. Gedde, MD
Download PDF
Sharon Revson* was in a panic. One Monday morning, the otherwise healthy 48-year-old suddenly noticed a gray, crescent-shaped scotoma across the superior visual field of her right eye. Over the next four days, as the scotoma expanded to encompass her entire visual field, she noted a new aching sensation around that eye.
As she had never experienced anything like this before, she rushed to her ophthalmologist. He found hand motion vision and an afferent pupillary defect (APD) in the affected eye, although the rest of the examination was normal. He immediately sent her to our neuro-ophthalmology clinic.
|
What's Your Diagnosis?
|
 |
|
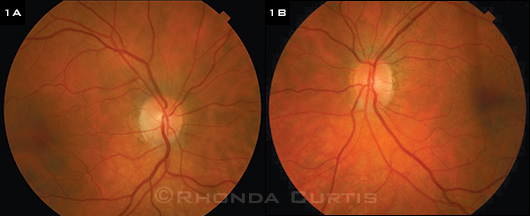
INITIAL EXAM. Both optic nerves appeared normal without swelling or pallor. The remainder of the posterior segment showed no inflammation or vasculitis.
|
We Get a Look
When we saw Ms. Revson, it was eight days after her symptoms had first appeared. Her best-corrected visual acuity (BCVA) was no light perception in her right eye and 20/20 in her left. The right eye demonstrated a nonreactive pupil with a marked APD; in contrast, the left eye showed normal pupil constriction to light and full color vision. Her motility was full bilaterally, but she reported increased pain in the right eye on far right gaze.
The anterior segment exam was remarkable for mild nuclear sclerotic cataracts and the absence of anterior chamber inflammation in both eyes. The fundus exam was normal bilaterally. Both optic nerves were normal without swelling or pallor (Figs. 1A and 1B). A Humphrey automated visual field (HVF) in the left eye was full.
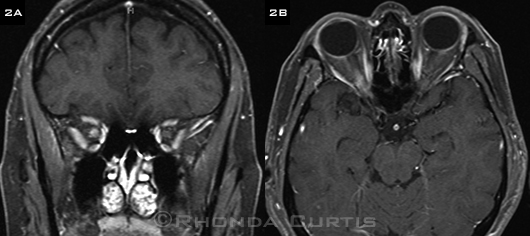
We ordered a magnetic resonance imaging (MRI) study of the brain and orbits with and without intravenous gadolinium contrast. The results showed enhancement and mild expansion of the right optic nerve at the orbital apex without evidence of a mass lesion, adjacent sinus disease, or meningeal enhancement (Figs. 2A and 2B).
 |
|
ADDITIONAL EVIDENCE. Coronal (2A) and axial (2B) T1-weighted fat-saturated postcontrast MRI images showed enhancement and mild expansion of the right optic nerve near the orbital apex. No other white matter abnormalities were present on review of the entire scan.
|
Making the Diagnosis
The presence of a right APD in the context of a normal fundus exam strongly suggested a process involving the retrobulbar optic nerve anterior to the optic chiasm. Clinical localization was corroborated by the MRI findings.
The differential diagnosis for painful vision loss with MRI evidence of an enhancing, expanded retrobulbar optic nerve is broad and includes demyelinating optic neuritis, inflammatory optic neuritis, infectious optic neuritis, and malignant infiltration of the optic nerve. It should be noted that although the term optic neuritis is often used synonymously with demyelinating optic neuritis, the two are not interchangeable. Optic neuritis is a broad term and encompasses inflammation of the optic nerve caused by a variety of conditions.
Demyelinating disease. Demyelinating optic neuritis can be isolated or occur in association with more widespread central nervous system demyelinating diseases such as multiple sclerosis (MS) and neuromyelitis optica (NMO). A brain MRI with IV contrast is useful in cases of presumed demyelinating optic neuritis to look for white matter changes suggestive of other demyelinating plaques.
Inflammatory disease. Inflammatory optic neuritis can occur in association with systemic inflammatory conditions such as sarcoidosis, systemic lupus erythematosus, Sjögren syndrome, inflammatory bowel disease, and granulomatosis with polyangiitis (formerly Wegener’s granulomatosis). A careful review of systems and focused lab studies can aid in diagnosis.
Infectious disease. Infectious causes of optic neuritis include syphilis, varicella-zoster virus, and tuberculosis.
Mimics. Neoplastic mimickers of optic neuritis such as lymphoma, high-grade optic nerve glioma, and metastatic carcinoma occur rarely; diagnosis often relies on clinical suspicion and optic nerve biopsy.
Pinning It Down
Lab work. For Ms. Revson, we ordered a serum angiotensin-converting enzyme level, fluorescent trepenomal antibody absorption, rapid plasma reagin, and a tuberculin skin test as well as the antinuclear antibody and Sjögren antibody tests; all results were normal. A computed tomography scan of the chest with contrast was unrevealing and found no evidence of sarcoidosis. We also ordered an antiaquaporin4 (AQP4) immunoglobulin G antibody test for NMO, but those results took a week to arrive.
Initial treatment. We initially admitted Ms. Revson to the hospital for three daily doses of 1 gram IV methylprednisolone. She was then discharged home on oral prednisone 60 mg daily. Despite a week of high-dose steroid treatment, her vision improved to only count fingers in the affected eye.
Readmission. One week after being drawn, Ms. Revson’s serum anti-AQP4 test returned markedly positive. She was readmitted for urgent plasmapheresis and completed five cycles over a 10-day period. Her prednisone was tapered to 20 mg every other day and long-term immunosuppression was initiated with mycophenolate.
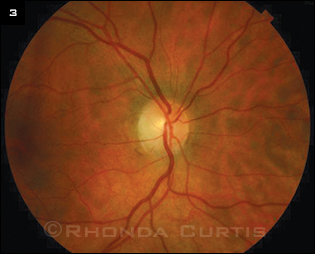
Resolution. Six weeks after her initial vision loss, Ms. Revson developed optic nerve pallor in the right eye (Fig. 3). Fortunately, four months after her vision loss, visual acuity improved to 20/30 and she identified 13 of 14 Ishihara color plates in the affected eye. HVF (30-2) testing showed only a few subtle points of depression in the temporal field. The left eye has remained unaffected, and she has suffered no additional demyelinating events to date.
 |
|
MANAGEMENT. Six weeks after initial presentation, the right optic nerve demonstrated temporal pallor.
|
Discussion
Once thought to be a form of MS, NMO is now known to be a clinically and pathologically distinct demyelinating disease characterized by autoantibodies to AQP4, a water channel protein found in astrocytes with higher expression in the optic nerve, spinal cord, and medulla.1
Demographics. Like MS, NMO is three times more common in females than in males, and the median age of presentation is about 35 (although age of onset varies along a wide spectrum).
Presentation and course. Approximately half of all NMO patients present with isolated optic neuritis; the remainder have isolated myelitis marked by symptoms of limb or trunk numbness, limb weakness, and bladder or bowel dysfunction. Only about 10 percent of cases present with classic simultaneous optic neuritis and myelitis. In patients who have only optic neuritis, subsequent myelitis can occur months to decades after the initial demyelinating event.
Unfortunately, NMO patients often demonstrate a relapsing course of severe optic neuritis with poor recovery leading to progressive disability.2 In fact, incomplete recovery is a hallmark feature of all central nervous system NMO attacks.
Potential for vision loss. NMO-associated demyelinating optic neuritis often presents with profound vision loss (less than 20/200), commonly reaches an acuity nadir of light perception or worse, and involves both eyes at onset in 20 percent of cases.3 In contrast, MS-associated demyelinating optic neuritis causes light perception vision or worse in only 6 percent of cases and is rarely simultaneously bilateral in adults.4 NMO should also be suspected in cases of optic neuritis with poor visual recovery.
Half of all patients who have had NMO for at least five years have vision worse than 20/200 in at least one eye. In comparison, patients with idiopathic demyelinating optic neuritis fare much better, with 92 percent of this group experiencing 20/40 or better vision in the affected eye 15 years later.5
As a structural correlate to this disparity in vision loss, optical coherence tomography analyses demonstrate greater retinal nerve fiber layer loss in NMO, with NMO patients experiencing an average loss of 30 to 40 µm, versus 10 to 20 µm in MS patients.3
Other symptoms. NMO patients may suffer intractable nausea, vomiting, or hiccups attributable to demyelination of the brain stem.
An urgent matter. As 25 percent or more of patients with clinical NMO will be anti-AQP4 seronegative, it is important to maintain a high index of clinical suspicion for optic neuritis that does not fit the classic presentation of demyelinating optic neuritis associated with MS. Patients with multiple clinical features of NMO but a negative anti-AQP4 antibody should be evaluated by a neurologist or neuro-ophthalmologist for consideration of long-term immunosuppression.
Early recognition of NMO is imperative to improve long-term morbidity, particularly given the risk of recurrent and severe relapses. Any delay in diagnosis or initiation of appropriate therapy may lead to blindness or paralysis for patients.
Treatment. Given the low prevalence of NMO, no standardized treatment trials exist. Nonetheless, patients with acute attacks are usually treated with a three- to five-day course of IV steroids. They are then usually maintained on oral steroids as they are transitioned to immunotherapy with medications such as azathioprine, mycophenolate, or rituximab.
The distinction between NMO and MS becomes especially important as immunomodulatory medications commonly used for MS—the platform interferon medications (such as Avonex and Betaseron), natalizumab, and fingolimod—not only fail to reduce NMO attacks but also can exacerbate the disease course.3
Although there are no evidence-based guidelines for the routine use of plasmapheresis in NMO, it has been shown to improve visual outcome in patients with steroid-refractory optic neuritis. Given the poor recovery of most NMO attacks in patients treated with steroids alone, early plasmapheresis may play a role in the acute treatment of all NMO demyelinating events in the future.
Conclusion
NMO is an important consideration in cases of seemingly isolated optic neuritis that present with profound vision loss (less than 20/200). Additional features suggestive of NMO include bilateral simultaneous optic neuritis, concomitant myelitis, recurrent optic neuritis, optic neuritis with poor visual recovery, or concomitant medullary symptoms (such as intractable vomiting or hiccups). These findings should trigger anti-AQP4 testing and/or referral to an appropriate neurologist or neuro-ophthalmologist. Early diagnosis and initiation of immunosuppressive treatment are paramount to optimizing long-term outcomes.
___________________________
* Patient’s name is fictitious.
___________________________
1 Galetta SL. J Neuroopthalmol. 2012;32(2):99-101.
2 Lai C et al. J Neuroophthalmol. 2011;31(1):16-19.
3 Morrow MJ, Wingerchuk D. J Neuroophthalmol. 2012;32(2):154-166.
4 Optic Neuritis Study Group. Arch Ophthalmol. 1991;109(12):1673-1678.
5 Optic Neuritis Study Group. Ophthalmology. 2008;115(6):1079-1082.
___________________________
Dr. Bei is a second-year ophthalmology resident, and Dr. McClelland is an assistant professor of neuro-ophthalmology; both are with the department of ophthalmology at Washington University in St. Louis. The authors report no relevant financial interests.