Download PDF
If you have yet to see a case of methicillin-resistant Staphylococcus aureus (MRSA) in your practice, you will soon enough—unfortunately. In the United States, MRSA now accounts for more than 30 percent of all serious S. aureus ocular infections, and the incidence is rising annually. Furthermore, over 80 percent of MRSA strains are resistant to all fluoroquinolones, a class of antibiotics that has been a mainstay in ophthalmology for the past two decades.1,2
The profile of MRSA has been changing rapidly over the past decade. Although health care–associated MRSA (HA-MRSA) was first recognized in the 1960s, community-associated MRSA (CA-MRSA), which was little known until the 1990s, now accounts for a growing proportion of cases. Some studies have found that it causes more than half of MRSA soft tissue infections,3 and an even higher prevalence is found in ocular infections.4-6 Because CA-MRSA lacks the clear risk factors and epidemiology seen with HA-MRSA, it may be underrecognized. Regardless of whether it’s HA or CA, however, MRSA ocular infection is on the rise. But ophthalmologists can take steps to help hold the line against the continuing expansion of antibiotic resistance.
|
MRSA Manifestation
|
 |
|
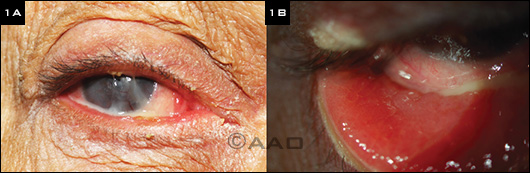
(1A) Chronic MRSA keratoconjunctivitis with blepharitis, mucopurulent discharge, and corneal scarring. (1B) Severe conjunctival papillary reaction.
|
How Big a Problem Is MRSA Really?
There seems to be a disparity between what the data show regarding the incidence and prevalence of MRSA and what community practitioners are experiencing, raising the question of how big a problem MRSA really is in ophthalmology.
In vitro vs. in vivo. According to David G. Hwang, MD, at the University of California, San Francisco, this difference may be the result of a disconnect between in vitro and in vivo response. He noted that increased in vitro resistance doesn’t necessarily mean that a drug will fail in clinical use. “In ophthalmology, when we have resistance, it’s often at a level that we can overcome with intensive topical therapy.” However, he added, “This does not mean it’s a nonissue. We see in experimental models of corneal infection that as you use strains with reduced susceptibility to a particular antibiotic, there’s a corresponding fall in the probability that antibiotic prophylaxis and treatment will be effective. There’s a direct, quantitative correlation.”
In his own practice, Dr. Hwang has seen a clear rise in resistance: “Even four or so years ago, I would see only a handful of MRSA cases each year, and now I see such cases routinely every few weeks; it has moved from a theoretical concern to the actual.” He recommended that clinicians consult surveillance data to detect emerging antibiotic resistance; this can provide a clearer picture of the trends than might be apparent from the smaller number of treatment failure cases encountered in an individual practice. (See “Sources for Surveillance Data.”)
Geographic factors. “Geography has a lot to do with prevalence,” noted Vikram D. Durairaj, MD, at the University of Colorado in Denver. “The types and numbers of MRSA infections that we see here in Denver might be different than in, say, Dallas or San Francisco. It’s so important to know your local surveillance data so that the antibiotics you use cover the bugs endemic to your area.” So far, the spread of MRSA has mirrored the geographic patterns we saw decades ago with penicillin resistance, said Preston H. Blomquist, MD, at University of Texas Southwestern Medical Center.
Dr. Hwang likens the naysayers to people who doubt climate change—an “I’m not seeing it in my backyard” point of view. “You just have to look at the recent surveillance data for ophthalmic infections as well as parallel trends for systemic infections to realize that MRSA is a very real and serious emerging problem for ophthalmologists,” Dr. Hwang said. “As ophthalmologists, we have a great advantage in treating eye infections because the accessibility of the ocular surface allows us to achieve high concentrations of antibiotics, which in many cases can overcome emerging resistance. That helps to delay, but not prevent, the appearance of resistance. But, on the flip side, as a niche market, we have a much harder time recruiting and developing new antimicrobials designed for ocular use.” Of the systemic antibiotics introduced in the past 20 years, only a small handful have been further developed for ophthalmic use, he said.
Sources for Surveillance Data
Where can you find data on emerging antibiotic resistance? A good place to start is the website for the U.S. Centers for Disease Control’s surveillance systems , which links with many other federal infectious disease resources.
Dr. Hwang recommends the University of Pittsburgh’s Campbell Eye Microbiology Lab. Although this is a tertiary referral lab in the Northeast, which potentially limits the generalizability of the data, he said, “They update the website regularly, archive data for longitudinal trend mapping, and, most important, provide data relevant to ocular isolates.”
At the local level. Although local susceptibility patterns can provide guidance for effective treatment, there is not yet a single, simple way to find such data.
Start with your own institution. If you are affiliated with an academic institution or large health care system, consult with the infectious disease department. Even if the department does not have a formal surveillance program, the specialists there can provide anecdotal guidance on what they are seeing.
Check your government agencies. Many public health departments track emerging infections and antibiotic resistance, though this varies widely by location. Contact your county or state department to learn what resources are available.
Consider a commercial entity. Some contract research organizations and laboratories produce customized reports for a fee. For example, one such entity, The Surveillance Network, claims that it collects strain-specific, antimicrobial resistance test results daily from sites across the United States.
|
How CA-MRSA Is Different
The most obvious difference between HA- and CA-MRSA is in demographics. Patients with CA-MRSA have no known connections with a health care institution, they are often otherwise healthy, and they are younger. For example, in one study, the median ages of CA- versus HA-MRSA patients were 39 years and 54 years, respectively.7 Clusters of CA-MRSA sometimes occur in sports teams, military personnel, and prison inmates.8
Different toxins. In addition, CA-MRSA is associated with Panton-Valentine leukocidin (PVL), a staphylococcal toxin that causes tissue necrosis and leukocyte destruction and has been linked to recurrent, severe primary skin infections. The pvl genes that encode for this toxin are rarely found in methicillin-sensitive S. aureus or HA-MRSA.3
PVL appears to be a major factor in the production of abscesses of the skin and eyelids in CA-MRSA infection. Thus, it was not surprising that in studies looking at patients with ophthalmic MRSA in a health care system in Dallas, 86 percent of those with MRSA preseptal cellulitis and/or lid abscesses had CA-MRSA.4,5
Possible different manifestations. Although earlier studies reported conjunctivitis as the most common ophthalmic manifestation of MRSA, the Dallas study found that preseptal cellulitis was the most common presentation from 2000 to 2009,4,5 followed by conjunctivitis, corneal ulcer, endophthalmitis, and orbital cellulitis. (See next month’s EyeNet for more on MRSA-associated cellulitis.)
Some different susceptibilities. Dr. Blomquist said, “The good news is that, unlike nosocomial [HA-] MRSA, which is multidrug resistant, CA-MRSA is still susceptible to several older antibiotics.” He emphasized that “one need not jump to vancomycin initially for nonsevere infections.” For example, his oral therapy regimen for preseptal cellulitis is double-strength trimethoprim/sulfamethoxazole (two tablets twice daily), plus or minus rifampin.
Countering Antibiotic Resistance
“We’ve been riding the fluoroquinolone bandwagon for about 20 years, and it’s been a great ride,” said Dr. Hwang, “but we now have a pathogen that is fundamentally in a fluoroquinolone coverage hole.” Indeed, a high percentage of both CA- and HA-MRSA strains are resistant to fluoroquinolones, even the newest generation, and this class of drug cannot be consistently relied upon for effective treatment of established MRSA infections.
“Ophthalmology has far fewer available antimicrobials than other areas of medicine, and there’s little to nothing in the way of new antimicrobial classes coming down the pike. We need to preserve vancomycin, our agent of last resort, and take steps to slow antibiotic resistance,” said Dr. Hwang. Following are some strategies to decelerate the progress of resistance.
Rotate, don’t repeat. Data now show that prior use of fluoroquinolones increases the risk of fluoroquinolone resistance, said Dr. Hwang. Thus, chronic use of fluoroquinolones is not recommended. “If prolonged treatment is necessary, it’s better to rotate your drugs than to repeat them.”
Use classic culture-directed therapy when possible. Antibiotic use should be appropriately limited, employing narrower-spectrum agents whenever possible, especially for mild or self-limiting infections. Determining which antibiotics are most effective in a given geographic area requires data. “It is so important to support nascent efforts to track ophthalmic susceptibility trends across different regions,” said Dr. Hwang.
Don’t forget the older agents. “You don’t always have to use the latest drugs,” Dr. Hwang said. Newer antimicrobials are not necessarily better than older ones. Trimethoprim–polymyxin B, for example, provides excellent coverage for over 95 percent of MRSA strains and over 90 percent of methicillin-resistant coagulase-negative staphylococci strains.9
Combine drugs. Using antibiotics in combination may forestall the development of resistance. Combination therapy is typically reserved for treatment or prophylaxis of serious infections potentially caused by resistant microbes.
Dose effectively. “When using antimicrobials, you have to achieve sufficient doses, not only to clear the infection but also to hit the concentration that prevents resistance from developing,” explained Dr. Hwang. “Dose at high levels for a limited period—hit hard, then get out.”
Choose antisepsis for prophylaxis and milder infections. “Disinfectants not only have the advantage of acting across broad classes of pathogens but they also avoid the problem of resistance,” said Dr. Hwang. They provide excellent infection control for surgical prophylaxis, given that the microbes are present in relatively small numbers. “Antiseptic strategies, using agents such as povidone-iodine, can be an effective approach,” said Dr. Hwang. In addition, these agents are generally less expensive than antibiotics.
Don’t waste vancomycin on prophylaxis! Although vancomycin is commonly used for routine prophylaxis for cataract surgery, Dr. Blomquist said, “I think that’s an unwise use; we need to save vancomycin for the biggest battles!” Drs. Hwang and Durairaj agreed with this point of view. Dr. Blomquist noted one exception, however: “I do use vancomycin prophylactically for open-globe injuries, but that’s about the only time that I do.”
___________________________
MRSA Ophthalmic Infection, Part 2—A closer look at MRSA-associated orbital cellulitis will appear in next month’s EyeNet.
___________________________
1 Asbell PA et al. J Cataract Refract Surg. 2008;34(5):814-818.
2 Haas W et al. Am J Ophthalmol. 2011;152(4):567-574.
3 Rutar T et al. Ophthalmology. 2006;113(8):1455-1462.
4 Blomquist PH. Trans Am Ophthalmol Soc. 2006;104:322-345.
5 Kruger M et al. Retrospective review of ophthalmic MRSA infections from 2005-2009 at Parkland Memorial Hospital. Presented at: Association for Research in Vision and Ophthalmology Annual Meeting; May 2, 2011; Fort Lauderdale, Fla.
6 Walvick MD, Khan A. Adv Biosci Biotechnol. 2013;4(2A):263-265.
7 Huang H et al. J Clin Microbiol. 2006;44(7):2423-2427.
8 Weber JT. Clin Infect Dis. 2005;41(suppl 4):S269-S272.
9 Asbell PA et al. Am J Ophthalmol. 2008;45(6):951-958.
___________________________
Preston H. Blomquist, MD, is Dr. W. Maxwell Thomas Chair in Ophthalmology, professor and vice chair for education, and residency program director for the department of ophthalmology at University of Texas Southwestern Medical Center. Financial disclosure: None. Vikram D. Durairaj, MD, is professor of ophthalmology and otolaryngology and chief of oculoplastic and orbital surgery at the University of Colorado Denver School of Medicine. Financial disclosure: None. David G. Hwang, MD, is professor of ophthalmology, codirector of the cornea service, and director of the refractive surgery service at the University of California, San Francisco. Financial disclosure: None.