Download PDF
Achieving a target refractive outcome is an essential—and complex—aspect of cataract surgery. “Our patients and peers are judging us by our refractive outcomes,” said Warren E. Hill, MD. “And we all think we’re doing really good cataract surgery, but the one true yardstick we have to measure our success is how well we are hitting the target.”
Unfortunately, many surgeons aren’t achieving optimal refractive accuracies, said Dr. Hill, at East Valley Ophthalmology in Mesa, Arizona. In a review of more than 260,000 eyes, he found that less than 1% of cataract surgeons attained a ± 0.50 D accuracy of 92% or better. The great majority were clustered around the 78% level.1
Is another formula the solution? It depends. You certainly don’t want to be left behind in the race toward accuracy. You also don’t want to jump off a formula if it’s working well in your practice. How should you evaluate—or reevaluate—your progress with lens power prediction?
Five Categories
To start, Douglas D. Koch, MD, suggested that clinicians familiarize themselves with the basics of IOL formula classifications. “The challenge for the clinician is determining what formula to use and when to change,” said Dr. Koch, at Baylor College of Medicine in Houston. “Most of the newer biometers have access to not only the traditional formulas but also the newer ones—so you can actually compare them on your own. But it’s most helpful if you’re able to understand how these formulas work so you can avoid any confusion about the advantages that each provides.”
Dr. Koch eschews the generational labels typically used to classify formulas (first-generation, second-generation, etc.). Instead, he prefers to group them by how they calculate IOL power and by the data they use:
1. Historical. “These include the very first refraction-based formulas, which might include a simple calculation such as IOL power = (1.25 × preoperative spherical equivalent),” said Dr. Koch. “They are obviously obsolete.”
2. Regression. These empiric formulas are based on regression analysis rather than the use of theoretical optics and eye modeling. They work by averaging a large number of refractive results. The most popular is the Sanders-Retzlaff-Kraff (SRK) formula, which was developed in the early 1980s.
3. Vergence. Vergence calculations are based on geometric optics. They are used to accurately estimate the effective lens position (ELP) and are further subclassified by the number of biometry variables used to predict this ELP. The two-variable formulas such as the Holladay 1 employ axial length and corneal curvature; the three-variable Haigis formula also uses anterior chamber depth; and the latest five- and seven-variable versions, such as the Barrett and Holladay 2, include lens thickness and corneal diameter. The Holladay 2 also includes age and refraction.
4. Artificial intelligence. Formulas such as the Hill-RBF (radial basis function) crunch outcome data from more than 10,000 cases using statistical modeling to identify relationships and patterns not found in the theoretical approaches above. Removed from the limitations inherent in ELP estimation, the result isn’t a specific equation per se, but rather a big data–driven approach that provides boundaries for prediction dependability.
5. Ray tracing. Similar to the theoretical versions above in their dependence on ELP, methods like the Olsen formula use individual rays that refract light on all surfaces of the lens and cornea. This allows the formula to take corneal and IOL higher-order aberrations into account, thus improving accuracy.
 |
|
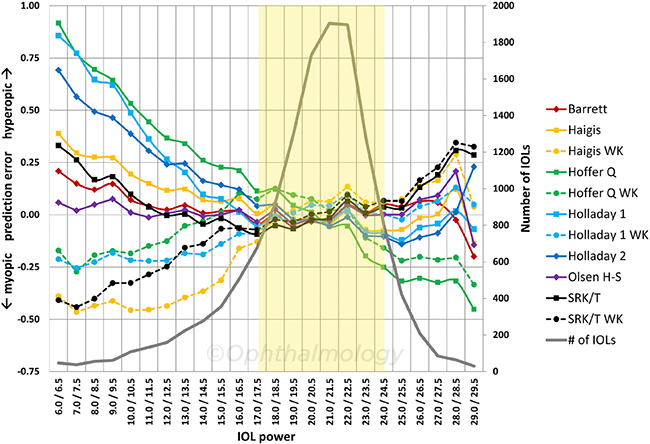
COMPARISON. Graph of prediction error versus IOL implant power for various formulas. The yellow area shows that for the majority of implants in the middle power range, all of the formulas perform equally well. The newer formulas distinguish themselves in the unusual eyes (such as those with short or long axial length). Modified from Fig. 5, Melles RB et al. Ophthalmology. 2018;125(2):169-178.
|
Time to Make a Change?
“It’s really up to the ophthalmologist to make a determination if change is needed in their practice,” said Jack T. Holladay, MD, MSEE, FACS, at Baylor College of Medicine in Houston. He suggested reviewing a sample of your latest 100 to 500 outcomes—including your recent refractive surprises. “You should know the percentage of your cases that are within plus or minus half a diopter accuracy. Compare the last 50 or so. If you’re below 75%, then you’re in the bottom percentile.” And if that’s the case, he said, you should be making changes to improve the predictability of your refractive outcomes.
Are new formulas the solution? “We’re approaching perfection in the accuracy of the newest power calculations and so the innovative leaps are getting smaller and smaller,” said Ronald B. Melles, MD, at Kaiser Permanente in Redwood City, California. All formulas perform equally well in the average eyes that represent 80% of cataract surgery, he added. But what sets the newer formulas apart is their performance in those 20% of cases that are unusual, such as short and long eyes.2,3
Equipment issues. Of course, the formula you choose will depend in part on the equipment you’re currently using. For instance, biometers that don’t measure lens thickness won’t allow you to take advantage of the Barrett or Olsen formulas, but they will allow for use of the Hill-RBF formula.
Evaluate the research. Practical limitations aside, reading independent reviews of new formulas is important, said Dr. Melles. He also noted that clinicians need not feel pressured into trying every formula that comes out. “Many formulas do very well in the dataset in which they were developed but not as well in external validation studies.” Given this limitation, he said, it’s important for clinicians to pay attention to those formulas that have been vetted with large datasets in peer-reviewed publications.2,3
Dr. Holladay agreed. “I’ve never seen an article where the author of a new formula didn’t always have the formula.” Sometimes, with the initial introduction, he said, “The formula looks like it’s the cream of the crop when, in fact, a subsequent review of the formula will include a more comprehensive analysis—and voilà, the performance of the calculations has weakened. You should be looking for independent, prospective studies that include all cases, all aberrations. Those are the ones that really count.”
Evaluate your own results. If you prefer not to take others’ work on faith, you can review a series of your own patient outcomes using different formulas, said Dr. Koch. “Certainly, new formulas like the Barrett Universal II and the Hill-RBF have been well validated with good large datasets, and they demonstrate high performance under a wide range of circumstances. They are two of the more superior formulas, and many ophthalmologists will just transition to those without hesitation.”
However, Dr. Koch said, “that’s the simple solution—the path of least resistance. If I’m a clinician in practice, I’d also want to run any new formulas in parallel and begin to look at my own data as a reference before trying [the formula] out in real patients.” Performing such a parallel assessment will allow the clinician to see how well these formulas do in complex eyes, he said.
Dr. Melles recommends focusing particularly on long or short eyes or those eyes that have resulted in refractive surprise. “Take those patients who have already had cataract surgery and postoperative refraction and who have unusually shaped eyes. Are the predictions of the new formulas under evaluation closer to the actual refractive outcomes that you achieved? Are they the same as your standard go-to formula? Going through these motions to familiarize yourself with new calculation models will give you a better sense of how they work, as well as their nuances.”
 |
|
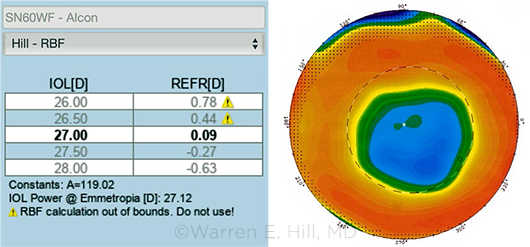
POST-LASIK CHALLENGE. This Pentacam image is from a patient who had –7.0 D of myopia before undergoing LASIK. This patient underwent early-generation LASIK technology (note the relatively strong demarcation between the treatment area and the surrounding, more normal, cornea). These somewhat older patients are now coming to cataract surgery.
|
 |
|
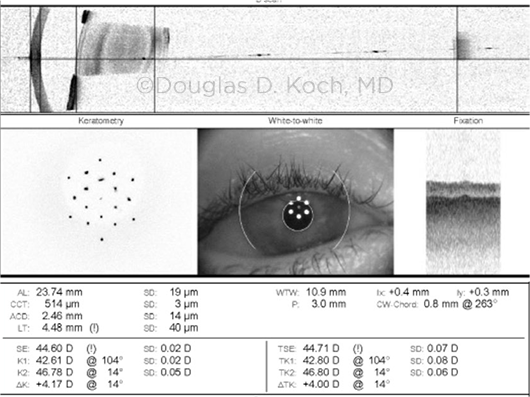
CORNEAL SURFACE CHALLENGE. This swept-source OCT biometry image shows poor-quality LED mires; this indicates corneal surface issues —e.g., dry eye, epithelial basement membrane disease—that must be addressed in order to obtain accurate corneal measurements.
|
Why Measurements Matter
The process of maximizing your refractive outcomes involves much more than which formula you choose. While all of the newer ones work, said Dr. Hill, the real key to success is beginning with the best measurements possible. “This discussion about IOL calculations isn’t about buying into the latest product out there. It’s about proper training.”
Garbage in … If you put noisy data into any formula, you can only expect to get less than optimal results, Dr. Hill pointed out. “The absolute, most fundamental aspect of IOL calculations is knowing how to take the measurements correctly—they have to be precise for these formulas to perform at their top level. If there’s a lot of mathematical noise in your measuring process, you won’t be able to tell the difference between many different calculation methods. It’s like looking at an image through a fog.”
Dr. Holladay agreed. “There’s nothing new in IOL formulas. Most of the new reporting is simply hype. The recent formulas perform at a high level; otherwise, nobody would use them. But it doesn’t make any difference what formula you use to maximize your refractive outcomes because the formula is rarely the problem or the limiting factor. It’s all about the precision and tolerances of your measurements and identifying any errors before going into cataract surgery.”
 |
|
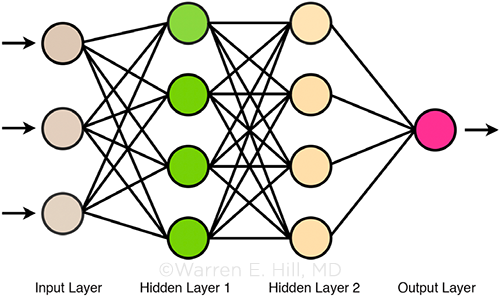
AI GUIDANCE. Artificial intelligence (AI) is expected to bring increasing sophistication to the process of IOL power selection. This image illustrates how an AI neural network operates: There is an input layer containing an organized pattern of data, one or more hidden layers that produce an output using an activation function (in the case of the Hill-RBF formula, radial basis functions), and an output layer that provides the final result(s).
|
Key Strategies for Better Measurements
Better biometry. Accurate biometry is one of the most important steps in calculating IOL power, said Dr. Melles. “We are getting to the point where the majority of error in refractive prediction comes not from the formula used, but from the biometry measurements. So careful biometry using an up-to-date platform is probably the most critical step to getting the best outcomes.”
The latest devices will give you better and more reliable data, Dr. Koch noted. “There are probably as many, or more, errors made with poor corneal measurements than with any other parameter—in fact, they’re probably a greater source of error than the formulas themselves.”
Biometry + topography. Dr. Koch added, “We know the importance of having a healthy corneal surface. And for that reason, it’s really important to have not only a good biometer but also some type of topographer, so that you can have two devices comparing corneal power and the steep corneal meridian.” In particular, he said, Placido topography provides a good assessment of tear film health so that the clinician can identify and address an ocular surface problem before proceeding with the lens calculation.
Better validation. How does a clinician know when a measurement is likely to be correct or incorrect? After all, said Dr. Hill, a measurement is only as good as your ability to know what it means.
Train your staff. “Too many of us have adopted a philosophy of ‘automate and delegate,’ letting staff run the show,” Dr. Hill said. “We can’t, however, replace careful thinking with simple button pushing and then plugging in numbers.”
He added, “Ophthalmologists are obviously very busy, but is it the best decision to shift the entire responsibility of preoperative measurements and lens calculations to your staff? Should you accept their results at face value while you simply select a lens? There has to be some degree of careful oversight by the surgeon. If we’re to be judged by our refractive outcomes, we must be the expert in the room and provide guidance and direction as well as the final decision-making.”
This involves investing in proper training and establishing meticulous processes, Dr. Melles said. In many cases, staff may not receive any formal training in proper biometry, or perhaps they were trained by previous colleagues who also lacked the training. “But that’s a red flag right there,” he said. “You need the very best technicians, and you need to make sure they are kept current.”
For instance, he said, “You need to emphasize that the eye should be lubricated before biometry to achieve an optimal measurement. The only way your staff will understand the importance of good patient outcomes is if the ophthalmologist is the person paying attention to and communicating the fine details.”
Screen your patients. Dr. Holladay also suggested implementing a set of screening techniques to help your technicians identify those patients who are likely candidates for refractive surprise.
“The set I use is simple,” he said. “First, your optical keratometry provides a standard deviation for the measurements taken—this should be less than ± 0.20 D (or ± 0.03 mm or ± 30 μm). If it’s not, the patient has irregular astigmatism and the measurement shouldn’t be trusted.” Second, the axial length should have a signal-to-noise ratio of greater than 2. Otherwise, said Dr. Holladay, the signal the machine is receiving from the reflection of the axial length is contaminated by significant noise due to a dense cataract or large posterior subcapsular cataract.
“Finally, measure both eyes and determine asymmetry,” Dr. Holladay said. “If the difference in axial length is greater than 0.3 mm, the difference in K value is greater than 1.0 D, or the difference in IOL power for the same target is greater than 1.0 D, recheck the values because they are highly unusual and likely inaccurate.”
Validate your measurements. Another common practice is the use of validation criteria available with some of the more popular biometers.4
“If you have a wrong measurement or there’s something else curious like an unusual anterior chamber, you need to be able to identify that immediately,” Dr. Hill said.
He pointed out that the device manufacturers have already developed the criteria. “Biometers like the Lenstar (Haag-Streit) and the IOLMaster (Carl Zeiss) provide you with a preflight checklist. These are surgical planning guidelines to help you determine when to flag something questionable and when to delete and repeat a measurement.”
Better lens constants. Optimizing and personalizing your own lens constant is an essential aspect of improving your refractive outcomes, said Dr. Holladay. You can improve your power calculation results by up to 5% in the process.
“Keep in mind that the manufacturers’ lens constants can be more than 0.5 D different than the clinician’s own,” he said. “That’s because they’re based on 20 to 30 surgeons all using different A-scans, different optical biometers, and different techniques for putting the lens in the bag. It can really throw you off.”
Better outcome tracking. You can’t adequately optimize your lens constant without first keeping track of your refractive outcomes—something that is overlooked by far too many ophthalmologists, said Dr. Hill. “It’s like going to sea without a chart or without a rudder.”
He added, “If you don’t monitor and keep consistent track of your refractive outcomes, how else will you know how you’re doing? How can you identify your trends, your successes, and your failures? How can you make the necessary adjustments to prevent a refractive surprise?”
Ideally, the clinician should set a goal of achieving refractive success in the 0.5 D range for nine out of 10 cases, said Dr. Hill. “I tell ophthalmologists all the time, move into this century—literally. Old formulas are just that, old formulas. And many practices are still using them—obviously not because they perform better, but because of inertia and casualness. They just don’t want to change or haven’t gotten around to changing yet.”
In contrast, your outcomes can be outstanding if you’re proactive, Dr. Hill said. “The practices with the most amazing outcomes in the United States are using the same equipment you likely have access to. But what separates them from everybody else is their attention to detail and knowing how to get the measurements right, regardless of which new formula is in use.”
___________________________
1 Koch DD et al. J Cataract Refract Surg. 2017;43(6):717-718.
2 Melles RB et al. Ophthalmology. 2018;125(2):169-178.
3 Melles RB et al. Ophthalmology. 2019;126(9):126(9):1335-1336.
4 Hill WE et al. J Cataract Refract Surg. 2017;43(7):869-870.
Disrupting the Status Quo
The idea of a universal IOL power formula that works in eyes of all shapes and sizes is an attractive one, but it is likely unrealistic for now, said Dr. Koch. “To build a universal formula means that we’re going to have to come up with a formula that truly does an almost perfect job of predicting the ELP, and no formula yet has demonstrated that type of perfection. Until we sort that out—or get around it with further advances in artificial intelligence formulas like the Hill-RBF—I like using four formulas (Holladay 1 and 2, Hill-RBF, and Barrett). We are still learning!”
What is clear, though, is that companies are developing new products that have the potential to completely transform the way clinicians go about IOL power calculation. These products involve “after-the-fact corrections,” Dr. Melles pointed out. In particular, he cited IOLs that can be adjusted after cataract surgery.
As Dr. Melles noted, “If you can easily measure and adjust refractive error postoperatively, an ophthalmologist might just need to get close enough in their pre-op predictions and fine-tune later.”
In the pipeline. Two leading possibilities are as follows:
The light-adjustable lens (RxSight). This photosensitive IOL was approved by the FDA in 2017 but is not yet available commercially in the United States. With the IOL in place, the surgeon reshapes the lens curvature and modifies its molecular structure using an ultraviolet light treatment to make postoperative adjustments to IOL power for the correction of minor refractive errors.
Refractive index shaping (Clerio Vision and Perfect Lens). This type of technology uses a minimally invasive, low-power femtosecond laser to change the refractive index of an already existing IOL without measurably changing its shape. Because each treatment affects only a very thin layer within the IOL, multiple adjustments to the same lens are possible over time.
Looking ahead. With these new technologies, “the latest-and-greatest formulas won’t be the secret to success,” Dr. Holladay said. “These companies are producing quantum leaps in how ophthalmologists will be able to achieve refractive success that’s at or near target.”
|
Meet the Experts
Warren E. Hill, MD In private practice in Mesa, Ariz., and an adjunct professor of ophthalmology at Case Western Reserve University in Cleveland, Ohio. Relevant financial disclosures: Carl Zeiss: C; Haag-Streit: C,P,S.
Jack T. Holladay, MD, MSEE, FACS Clinical professor of ophthalmology at Baylor College of Medicine in Houston. Relevant financial disclosures: Carl Zeiss: C; RxSight: C,O.
Douglas D. Koch, MD Professor of ophthalmology and Allen, Mosbacher, and Law Chair in Ophthalmology at Baylor College of Medicine in Houston. Relevant financial disclosures: Carl Zeiss: C; Perfect Lens: C.
Ronald B. Melles, MD Ophthalmologist at Kaiser Permanente in Redwood City, Calif. Relevant financial disclosures: None.
Full Financial Disclosures
Dr. Hill Alcon: C; Carl Zeiss: C; Haag-Streit: C,P,S; Omega Ophthalmics: C,O; Optos: L.
Dr. Holladay AMO: C; AcuFocus: C,O; Alcon: C; ArcScan: C,O; Carl Zeiss: C; Elenza: C,O; MST: C; Oculus C; RxSight: C,O; Visiometrics: C,O.
Dr. Koch Alcon: C; CapsuLaser: C; Carl Zeiss: C; Ivantis: C,O; Johnson & Johnson: C; Perfect Lens: C; PowerVision: C,O; Vivior: C.
Dr. Melles None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|