Download PDF
To improve care for uveitis associated with juvenile idiopathic arthritis (JIA), an expert panel has issued comprehensive recommendations for the screening, monitoring, and treatment for uveitis in children with JIA.1,2
The American College of Rheumatology and the Arthritis Foundation convened the panel of ophthalmologists, rheumatologists, patient representatives, and informatics methodologists.
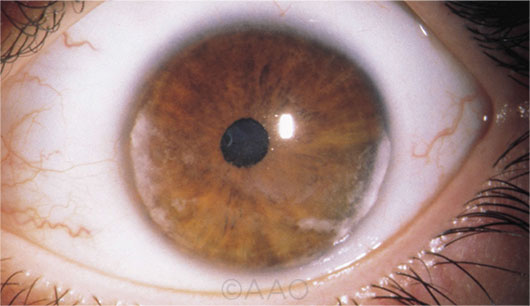 |
JUVENILE ARTHRITIS. Close monitoring is essential in patients with JIA, given the risks of chronic JIA-associated uveitis (seen here).
|
Urgently needed. The guidelines are an urgently needed tool for remedying deficiencies in the care that children with uveitis receive, said panel member Gary N. Holland, MD, at the University of California-Los Angeles and the Stein Eye Institute.
“There has been great variation in treatment practices, and it has been apparent to both uveitis specialists and pediatric rheumatologists that not all children are receiving optimum care. We often see pediatric patients who have been undertreated or whose treatment has been delayed,” Dr. Holland said.
“Some clinicians are not aware that uveitis in children with JIA is always a chronic disease needing long-term treatment. Uveitis will almost certainly recur if treatment is stopped immediately after initial control is achieved,” he said. “Also, some clinicians do not follow patients sufficiently often to identify exacerbations of inflammation or uveitic complications before they result in tissue damage and vision loss. Moreover, there may be a lack of familiarity with current drug options—especially with their use in children.”
Panel highlights. Noting the poor quality of the literature on JIA-associated uveitis, the panel said it had to combine the available, low-quality evidence (much of it in adults), observational data, and consensus expert opinion to develop the guidelines.
Some of the group’s recommendations for children and adolescents with JIA are as follows:
Frequent screening. Children at high risk for developing uveitis should undergo an ophthalmic screening every three months. High-risk groups are children who have certain types of arthritis (including psoriatic arthritis and oligoarthritis), those who were younger than seven years at JIA onset, and those who have had JIA for four years or less, the panel wrote.
Close monitoring of stable cases. If uveitis is under control, the panel strongly recommended that ophthalmic monitoring take place no less frequently than once every three months. Additional monitoring should take place within one to two months each time topical glucocorticoid dose or systemic therapy is altered.
Glucocorticoids in chronic anterior uveitis (CAU). For initial therapy, prednisolone acetate 1% topical drops are recommended over difluprednate topical drops. Frequent topical glucocorticoids should not be used as long-term treatment; instead, the panel recommended switching to a glucocorticoid-sparing immunosuppressive agent.
Systemic immunosuppression for active CAU. Treatment with subcutaneous methotrexate instead of oral methotrexate may be more effective. Addition of a tumor necrosis factor (TNF) inhibitor in severe cases should utilize one of the monoclonal antibodies (e.g., adalimumab or infliximab), because etanercept, a different type of anti-TNF agent, has not been found to be effective for control of uveitis.
Real-world reflections. Dr. Holland noted that the guidelines reflect information from various sources, not just from medical experts. “One of those sources was patients themselves, who expressed preferences among treatment options, making the guidelines applicable in real-world situations,” he said.
And although all children with chronic anterior uveitis would ideally be evaluated and treated medically in a team approach by a uveitis specialist and a pediatric rheumatologist, that is not always possible, Dr. Holland noted. “These guidelines will be especially useful for physicians outside of urban areas who may not see large numbers of children with uveitis and whose patients do not have easy access to pediatric rheumatologists or uveitis specialists.”
—Linda Roach
___________________________
1 Angeles-Han ST et al. Arthritis Care Res. 2019;71(6):703-716.
2 Angeles-Han ST et al. Arthritis Rheumatol. 2019;71(6):864-877.
___________________________
Relevant financial disclosures—Dr. Holland: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Holland None.
Dr. Kuriyan Alimera Sciences: C; Allergan: C; NEI: S; Regeneron: C; Roche/Genentech: S; Second Sight: S; Valeant: C.
Dr. Medeiros Allergan: C; Biogen: C; Carl Zeiss: C;S; Galimedix: C; Heidelberg Engineering: S; NEI: S; Ngoggle Diagnostics: P; Novartis: C; Reichert: C,S.
Dr. Peeler None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review