Download PDF
Which giant cell arteritis (GCA) patients might benefit from early treatment with agents other than conventional steroid therapy?
A recent study offers a quantitative approach to answering that question.1
In this retrospective study, researchers assessed 42 GCA patients who had undergone temporal arterial biopsies (TABs), the gold standard for GCA diagnosis, at Houston Methodist Hospital in 2015.
The researchers reviewed patient charts for 4 variables: recurrence, number of days on glucocorticoids, referral to a rheumatologist, and placement on immunomodulatory therapy (IMT). They then correlated patients with features of healing/treated GCA and the 4 variables to the CD68 macrophage immunohistochemical marker found on the TAB specimens.
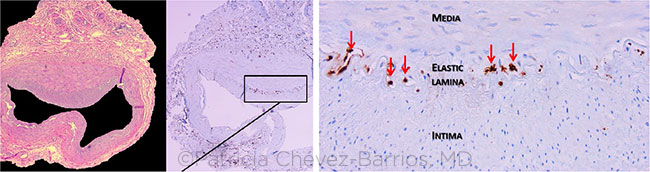 |
COMPARISON. Routine hematoxylin and eosin (HE) stain of a histopathologic section of a temporal artery (left) shows irregular intima hyperplasia but no discrete multinucleated giant cells. Immunohistochemistry using CD68 antibody discloses positive cells at the level of the elastic lamina located in the media (muscularis) side with at least 5 positive cells (center and right, arrows).
|
Slice metric. Using a metric of CD68+ cells per histologic slice, they found the following:
- Patients whose symptoms recurred at least once during follow-up had a greater number of CD68+ cells per slice compared to those with no recurrence (2.40 vs. 1.13, respectively).
- There was no statistical difference in cells/slice between patients who were referred to rheumatology and those who were not.
- Patients eventually placed on IMT had a greater number of cells/slice than did those who did not receive IMT (5.00 vs. 1.21, respectively).
“In this study, patients who had a more severe disease course [necessitating being placed on IMT] had a statistically significant greater number of CD68+ cells per slice than those patients not placed on IMT,” said Patricia Chévez-Barrios, MD, at Houston Methodist Hospital.
A surprise. As for time on glucocorticoids, the researchers had hypothesized that the number of CD68+ cells/slice would decline over the course of treatment. In fact, there was no correlation between cells/slice and length of time on glucocorticoids, even beyond 40 days following initial treatment.
Clinical implications. Since most patients are on glucocorticoids at the time of TAB, quantification of CD68+ cells in TAB may help to identify patients with recalcitrant disease who cannot be managed with steroids alone, Dr. Chévez-Barrios said.
She suggested that pathologists could employ the metric to aid in determining severity of the disease course. “If the patient has an increased number of CD68+ cells per slice—greater than 2 cells per slice—then the patient might need to be referred for rheumatologic treatment sooner than a patient who has fewer than 2 cells per slice.”
—Miriam Karmel
___________________________
1 Sultan H et al. Am J Ophthalmol. Published online June 8, 2018.
___________________________
Relevant financial disclosures—Dr. Chévez-Barrios: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Chévez-Barrios NASA: S.
Dr. Foster None.
Dr. Khawaja Allergan: C,L; Grafton Optical: L; Novartis: C; Thea: C.
Dr. Wirostko EyeGate: E;O.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review