As the Academy Joint Meeting approaches, EyeNet brings you a preview of some papers to be presented there. Each paper was chosen by its session chairperson because it either constitutes important news in the field or is illustrative of a trend. Although only five subspecialties are represented below, there also will be paper sessions for intraocular inflammation/uveitis, neuro-ophthalmology, ocular tumors/pathology, oculoplastics, and pediatric ophthalmology. Look for a complete list of papers in the Final Program (pages 153-172) or Pocket Guide (pages 89-93), or go to www.aao.org/annual-meeting/program.
Cataract Femtosecond Paper
The “Ideal” Capsulorrhexis —Does It Matter?
Skeptical that a femtosecond laser would make his cataract surgeries more predictable, Jonathan A. Davidorf, MD, decided to investigate the underlying premise—that a better capsulorrhexis equals more accurate postoperative refractions.
“There are abundant claims that a perfect capsulorrhexis will improve our outcomes,” said Dr. Davidorf, a refractive and cataract surgeon in private practice in West Hills, Calif. “But that begs the question: Does a perfect or near-perfect capsulorrhexis confer better refractive outcomes than a less perfect capsulorrhexis?
“I think we’re pretty good with our refractive outcomes in my practice. But if the refractive outcomes are better with a laser capsulotomy, I could be on board with that,” he said.
To help himself decide about acquiring a cataract laser, Dr. Davidorf reviewed videos of 175 consecutive cataract surgeries in his practice and gauged the impact of capsulorrhexis morphology on the intraocular lens (IOL) power calculations.
“These were older surgical videos. I wasn’t even thinking about trying to do the capsulorrhexis perfectly. I was just doing regular cataract surgery,” he said.
He classified the capsulorrhexes in three groups.
- Ideal: those surgeries in which the entire IOL edge had 0.25 to 0.75 mm of capsule overlap.
- Average: those with minor deviations from the ideal amount of overlap.
- Too little or too much overlap: those with less than 0.25 mm of overlap in at least 3 clock-hours of the IOL edge or with more than 0.75 mm of overlap in at least 3 clock-hours.
Based on the patients’ refractions at one month after surgery, Dr. Davidorf calculated the errors in predicted refraction and found that the mean predictive error in the entire cohort was +0.24 D ± 0.50 D (Haigis formula, optimized) and +0.25 ± 0.47 D (SRK-T).
In the “ideal” group, the mean error (Haigis) was +0.28 ± 0.44 D, compared with –0.03 D ± 0.35 D in the eyes with insufficient overlap (p = 0.01 Τ test and p = 0.46 ANOVA F test). With SRK-T, the difference between groups was about 0.1 D smaller, without statistical significance. (There was only one eye with excessive overlap, so no comparison was done.)
“If capsulorrhexis morphology is important, this is not evident after more than 100 random cataract surgeries,” Dr. Davidorf said. “In a series in which there was no preoccupation with creating 0.25 to 0.75 mm of overlap, there were very few eyes that deviated drastically from that. Presumably, we could manually create that configuration nearly every time if it were an intended goal.”
His take-home message from the study comes as another question. “If there’s no difference in refractive outcomes between the perfect capsulorrhexes and the poor ones, how are you going to tell me that there’s going to be a difference for the average ones?” he asked. “It’s possible that a perfect laser capsulotomy would get better results than a manual capsulorrhexis—it defies common sense, but it’s possible.”
—Linda Roach
___________________________
Dr. Davidorf is on the speakers bureau for Alcon and receives grant support from AMO.
| Impact of Capsulorrhexis Morphology on the Predictability of IOL Power Calculations. When: Sunday, Nov. 11, 10:27-10:34 a.m. during the first cataract femtosecond paper session (10:15 a.m.-noon). Where: Grand Ballroom S100c. Access: Free. (There also is a second cataract femtosecond paper session. When: Tuesday, Nov. 13, 8:30-10:15 a.m. Where: Room S406b. Access: Free.) |
Cornea Paper
Four Ways to Unfold the DMEK Graft
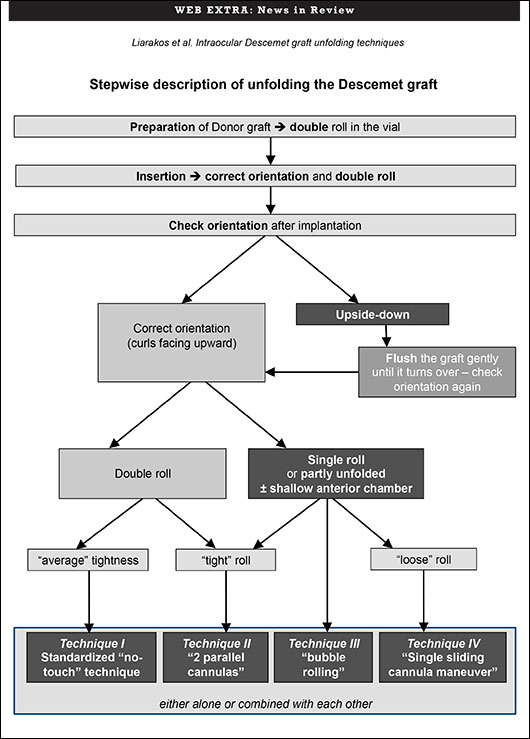
Take your pick. That’s the conclusion of a study evaluating four techniques for unfolding the ultrathin Descemet membrane graft during Descemet membrane endothelial keratoplasty (DMEK). None of the options compromised best-corrected visual acuity, endothelial cell density, or the six-month postoperative complication rate. The study involved 100 consecutive DMEK cases at the Netherlands Institute for Innovative Ocular Surgery (NIIOS).
“We wanted to identify and categorize various intraocular Descemet graft unfolding techniques, in order to describe different approaches and evaluate how these techniques correlated with the eventual clinical outcome and/or the incidence of postoperative complications,” said Vasilis S. Liarakos, MD, a cornea specialist at NIIOS who was doing a fellowship with DMEK pioneer Gerrit R. J. Melles, MD, PhD, at the time of the study. “The preferred technique or combination of unfolding techniques did not affect the final clinical outcome,” he said.
The study was conducted, in part, to address concerns over the feasibility of DMEK, particularly the unfolding of the Descemet graft inside the anterior chamber of the recipient eye, said Dr. Liarakos. Challenges to feasibility include unfolding in phakic eyes or in an anterior chamber with an IOL or glaucoma tube, he added. Once a DMEK graft is placed into the patient’s eye, it tends to curl up into a scroll. The scroll has to be unrolled, and the surgeon must determine which side should face the recipient cornea and which side should face the inside of the eye.
To address this challenge, Dr. Melles had developed a standardized “no-touch” technique for unfolding a “double Descemet roll.” The most essential step is performed before implantation. In a glass vial, a single roll of Descemet donor material is manipulated by irrigation with balanced salt solution until the graft can be unrolled and folded back, while forming two adjacent rolls. The double-rolled graft is then inserted into the eye and unfolded over the iris.
Three variations of the “no-touch” technique are intended for eyes with a less forgiving anatomy or when a double Descemet roll cannot be ideally achieved or maintained. In one variation, two cannulas unfold a single, or “tight,” Descemet roll. In a second, a small injected air bubble unfolds the Descemet roll. A third employs a single sliding cannula maneuver to unfold a loose Descemet graft in a shallow anterior chamber.
“The concept is not to choose any one technique but to choose the most suitable technique or combination of techniques for each situation,” Dr. Liarakos said. To that end, the NIIOS team created a decision tree indicating when to use each technique and why.
“The conclusion is that these different alternative techniques do not compromise the outcome,” Dr. Liarakos said. “Each technique may be used either alone or in various combinations with one other, adapting to the course of the operation.”
—Miriam Karmel
Dr. Liarakos reports no related financial interests.
___________________________
To view the techniques, visit www.youtube.com/watch?v=T2lAh_gKNiA. Or, see the decision tree below.
| Intraocular Graft Unfolding Techniques in Descemet Membrane Endothelial Keratoplasty. When: Monday, Nov. 12, 8:42-8:49 a.m., during the cornea, external disease paper session Part I (8:30-10:10 a.m.; Part II, 10:12 a.m.-noon). Where: Room S405. Access: Free. |
Glaucoma Paper
Devices May Be Less Costly Than Medication
A Canadian team is looking at minimally invasive glaucoma devices such as the iStent, Trabectome, and endoscopic cyclophotocoagulation (ECP) handpiece as an option to reduce dependency on topical medications for patients with mild or moderate open-angle glaucoma. Their findings indicate that these devices may be less costly for the patient—and the health care system—according to a study by Yiannis Iordanous, MD, and colleagues. Dr. Iordanous is a third-year ophthalmology resident at Western University in London, Ontario.
The researchers conducted a cost analysis of these devices projected over a six-year period and compared these figures with the costs of topical therapy. They found that the Trabectome yielded a $242.68 cost savings over medications, and ECP provided a $742.68 cost savings. The iStent was $57.32 more expensive under the scenarios studied, which did not include the start-up costs for any of the devices. The start-up cost would include purchasing the ECP and Trabectome consoles, which is not required for the iStent. These costs were excluded due to the high degree of variability by which their expenses are covered.
“What our findings tell us is despite the fact that these newer minimally invasive devices may appear to be more expensive up front, they may actually offer savings compared with the cost of long-term medication use,” Dr. Iordanous said.
“These findings are particularly relevant to ophthalmologists who work in a government-funded medical system where the health care system is looking to reduce costs,” added Dr. Iordanous. “This region is going through many changes, and if we want to bring new devices or treatment alternatives to our hospitals, it helps to show that they are not only beneficial to the patient but also offer cost savings to the health care system.”
Dr. Iordanous and his colleagues used published utilization rates and data from the Ontario Drug Benefit formulary to determine the average annual cost of glaucoma medications projected over a six-year period.
They calculated the per-patient cost of the glaucoma devices based on information from suppliers. These costs included the price of the implanted iStents, the cost of a procedure pack for the Trabectome, and the per-case cost of using the disposable ECP handpiece. They excluded the start-up capital costs of these devices, focusing on the costs associated with continued use of these devices extrapolated over six years.
“We also examined the possible cost savings under a variety of scenarios, including going from two medications to no medications after treatment with these devices,” Dr. Iordanous added.
While the results from this study illustrate the cost savings possible by utilizing these minimally invasive glaucoma devices, they do not tell the entire story. Dr. Iordanous said more research is needed to evaluate the effectiveness of the devices over the long term and their possible impact on the patient’s quality of life.
“We haven’t looked at the indirect costs of minimally invasive devices versus topical medications,” he said. “If we can determine the true cost-effectiveness of these treatment alternatives, then we can have a strong argument for using these devices as part of our glaucoma treatment paradigm.”
—Lori Baker Schena
___________________________
Dr. Iordanous reports no financial interests.
| Cost Comparison of the Trabectome, iStent, and Endoscopic Cyclophotocoagulation With Glaucoma Medication in the Ontario Health Insurance Plan. When: Tuesday, Nov. 13, 10:30-10:37 a.m., during the glaucoma paper session (10:30 a.m.-noon). Where: Room S405. Access: Free. (There also is an earlier glaucoma paper session. When: Sunday, Nov. 11, 10:15 a.m.-noon. Where: Room S405. Access: Free.) |
About the Best Papers in 2012
At the end of each paper session, the expert panel members will select one paper that they consider the best of that group. These Best Papers will be listed in the Saturday, Sunday, and Monday , which are sent out from the Joint Meeting, and in the January EyeNet.
|
Refractive Surgery Paper
LASIK vs. Contact Lenses: Satisfaction Study
Comparing patient satisfaction with two widely accepted measures of vision correction—LASIK and contact lenses—has never been done in a prospective study, according to Francis W. Price, MD, president of Price Vision Group in Indianapolis. “We wanted to set a new benchmark for how we evaluate LASIK, comparing it to contact lenses,” he said. In the fall of 2010, Dr. Price started a prospective, multicenter study that surveys participants annually for three years to assess visual satisfaction, side effects, and complications with the two means of correction.
Participants are between 18 and 60 years of age, with no keratoconus, abnormal corneal topography, or multifocal corrections. To date, 883 subjects are enrolled in the LASIK arm, and 610 in the contact lens arm. Sixty-one percent of patients are female; median age is 34; and median spherical equivalent is –3.5 D (range –11 D to +4 D).
Nineteen ophthalmic practices around the United States are participating. “What’s interesting is that the results are all based on patient perception, not on what the MD sees, which we think is very important,” said Dr. Price.
The baseline survey consists of multiple choice questions. Participants complete this online in the physician’s office, and the staff enters basic refraction data. Patients in the LASIK arm complete the baseline survey before undergoing the procedure. By the Joint Meeting, the researchers hope to have baseline responses from up to 1,700 enrollees and up to 500 responses to the one-year survey.
Survey questions cover the following areas:
- Patient satisfaction with the correction
- Visual problems (such as difficulties with night vision)
- Ocular symptoms (such as dry eyes)
Preliminary data from the baseline survey show that 95 percent of those who continued to wear contacts would recommend them to friends or family members, and 89 percent of contact lens wearers who were having LASIK would still recommend contact lens wear to others. Coauthor Marianne Price, PhD, said that the team anticipated a bigger difference in contact lens satisfaction between those who continued wearing them and those who chose LASIK.
“We think this is really exciting—a big population study with huge public health implications. Millions of people have had LASIK or wear contact lenses. The information will be very helpful for developing and marketing both types of correction—in terms of how to improve what we are doing,” said Dr. Francis Price.
—Laura B. Kaufman
___________________________
Drs. Francis and Marianne Price report no corporate sponsorships for this study. He is founder and president, and she is executive director, of the Cornea Research Foundation of America, a 501(c) organization. The survey is funded by the participating practices, the Cornea Research Foundation of America, and patient donations.
| Survey Study Comparing Satisfaction With LASIK and Contact Lenses for Vision Correction. When: Sunday, Nov. 11, 2-2:07 p.m., during the refractive surgery paper session (2-3:40 p.m.). Where: S405. Access: Free. |
Retina Paper
COPERNICUS at One Year
How early and how much? The first year of the phase 3 COPERNICUS trial began answering key questions like these about treating central retinal vein occlusion (CRVO) using intravitreal aflibercept injection (IAI), formerly known as VEGF Trap-Eye, now called Eylea.
In the first six months of the study, 114 patients were randomized to receive monthly injections of 2 mg IAI, while 73 received sham monthly injections. From week 24 on, however, both groups received 2 mg IAI as needed (PRN), based on both visual acuity and anatomic changes.
One question, said lead author David M. Brown, MD, in private practice with Retina Consultants of Houston, was whether it was possible to maintain the visual gains in the active treatment arm with PRN dosing alone. He said that unlike age-related macular degeneration, in which recurrent fluid can cause irreparable damage to the photoreceptors, it is more plausible that vision can be maintained in a vascular disease such as CRVO, which predominantly affects the inner retina.
With a mean gain of 17.3 letters in best-corrected visual acuity at 24 weeks achieved on monthly injections, patients in the active arm maintained a gain of 16.2 letters at week 52 after switching to PRN status. During the second six months of the trial, patients received an average of 2.7 treatments—about one every 68 days.
Although patients in the sham arm also gained vision with PRN treatment—7.8 letters between weeks 24 and 52 (for a net gain of 3.8 letters from week 0 to 52)—the improvement was not as substantial as in the active arm, which gained 13 letters on average by four weeks. “Even in the first month of PRN treatment, patients in the sham arm only gained about 3 letters,” said Dr. Brown, leading him to this conclusion: “Six months is probably too long to wait to initiate treatment.”
In the first six months of the trial, patients in the sham arm lost 4 letters. By contrast, this did not occur in the Lucentis CRUISE trial (monthly injections for six months of either 0.3 mg or 0.5 mg of Lucentis versus sham for treatment of macular edema following central retinal vein occlusion). However, in CRUISE, patients with relative afferent pupillary defect were excluded, said Dr. Brown, creating a patient pool with less sick eyes (by excluding those that probably had more ischemia).
In the COPERNICUS study, the sham group had three times more adverse events than did the treatment group. This was largely due to neovascularization from ischemia. There were no unexpected side effects overall, other than those common to treatment with an anti-VEGF, said Dr. Brown. “Almost all the side effects were related to putting a needle in the eye.”
Aflibercept offers a new mechanism of anti-VEGF action. It works like an antibody but instead is a fusion protein composed of two types of VEGF decoy receptors, said Dr. Brown. “This gives it higher binding affinity, allowing it to strongly trap VEGF and, possibly, extend dosing intervals. It also blocks placental growth factor, which is thought to be involved in angiogenesis.”
—Annie Stuart
___________________________
Dr. Brown is a consultant for Bayer, Genentech/Roche, and Regeneron.
| One-Year Results of the Phase 3 COPERNICUS Study: Intravitreal Aflibercept Injection in Central Retinal Vein Occlusion. When: Monday, Nov. 12, 2:12-2:19 p.m., during the retina paper session Part I (2-3:40 p.m.; Part II, 3:42-5:35 p.m.). Where: S406b. Access: Free. |