Acetazolamide & IIH: New Trial Results
For the first time, ophthalmologists have solid evidence to guide treatment of idiopathic intracranial hypertension (IIH), thanks to a multicenter randomized trial funded by the National Eye Institute and coordinated by the Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC).1 Formerly called pseudotumor cerebri, IIH is a disease that can cause disabling vision loss. It mainly affects overweight women.
Enrolling 165 participants with IIH and mild visual loss at 38 sites in North America, the trial compared a low-sodium weight-reduction diet plus the maximum tolerated dosage of oral acetazolamide (up to 4 g per day) or placebo for six months. The average body mass index at enrollment was 40, and all but four participants were women.
 |
|
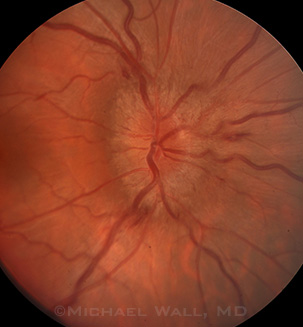
PAPILLEDEMA. Optic nerve swelling in patient with IIH.
|
Home-run outcomes. Best known as a glaucoma drug, acetazolamide has been commonly prescribed for IIH, despite a lack of supporting evidence. “This trial offers the first proof that acetazolamide improves visual outcomes in people with IIH,” said Michael Wall, MD, trial director and professor of neurology and ophthalmology at the University of Iowa. Further, he said that acetazolamide achieved statistical significance in reducing papilledema, improving quality of life, and lowering cerebrospinal fluid (CSF) pressure.
Overall, the mean improvement in perimetric mean deviation (PMD) was 0.71 dB greater with acetazolamide than with placebo, though it was substantially higher in patients with worse grades of papilledema at baseline. Although the treatment effect on PMD appears modest in patients with mild visual loss, said Dr. Wall, the vision-related quality-of-life measures improved significantly, suggesting that the effect is clinically meaningful.
Side effects. At high doses, acetazolamide can cause bothersome side effects such as fatigue, nausea, tingling in hands and feet, and a metallic taste in the mouth. However, trial participants tolerated the drug well, reaching an average daily dose of 2.5 grams a day. If side effects interfered with activities of daily living, the dosage was gradually stepped down, said Dr. Wall, and any adverse events reversed once the medication was stopped.
Weighty results. Weight loss has previously been shown to be effective for IIH, which was corroborated in this trial in both the acetazolamide and placebo groups. And because the drug itself can enhance weight loss, the researchers performed an analysis to tease apart the results. They found that the drug’s effects were independent of weight loss.
Recommendations. The researchers strongly advise the use of acetazolamide at the maximum tolerated dosage, coupled with a low-sodium weight-loss program, for patients with mild visual loss and moderate to marked papilledema. Patients who have grade 1 or 2 papilledema without visual loss can be managed with diet alone, said Dr. Wall, but should be followed closely and started on acetazolamide if needed.
“We can’t generalize findings of this trial to more severe cases,” said Dr. Wall, who noted that another trial is needed to compare a medical regimen with surgery to relieve pressure on the optic nerve. Studies are also needed to find the cause of IIH, which leads to a blockage in spinal fluid absorption and an increase in CSF pressure. Dr. Wall added, “We have lots of clues but, so far, not many
answers.”
—Annie Stuart
___________________________
1 Wall M et al. JAMA. 2014;311(16):1641-1651.
___________________________
Dr. Wall reports no related financial interests.
Horizons in Glaucoma
A Predictive Model to Personalize Follow-Up?
The mathematical power of a Kalman filter was good enough to put the Apollo 11 astronauts on the moon—could this forecasting method also help ophthalmologists decide how often to monitor patients with early to moderate glaucoma?
Quite possibly, according to researchers who applied the Kalman filter technique to several years of patient data from two landmark clinical trials of progression in patients with open-angle glaucoma (OAG). In their recent paper,1 the authors said that this “time to next test” (TNT) algorithm accurately predicted future values of intraocular pressure levels and visual field parameters.
If validated, the algorithm could enable clinicians to more quickly determine, case by case, the optimal time to perform the next set of glaucoma tests, said clinical leader Joshua D. Stein, MD, MS, assistant professor of ophthalmology and visual sciences at the University of Michigan.
 |
|
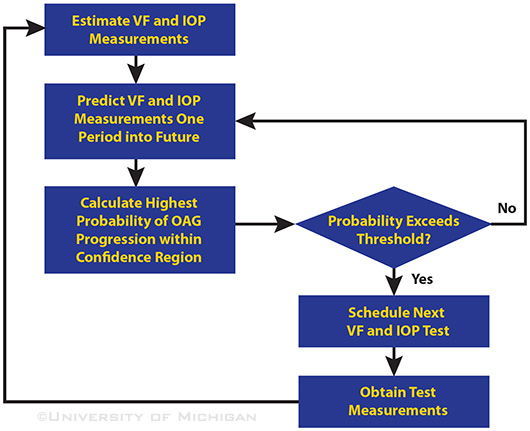
“TIME TO NEXT TEST” ALGORITHM. The Kalman filter estimates the current visual field (VF) parameters and intraocular pressure (IOP) measurements and predicts their future values, while the logistic regression estimates the highest probability of progression for those future values.
|
Personalized predictions. “Unlike existing algorithms that compare each patient to a normative database, the algorithm we developed learns about each patient by studying their past performance on these tests and couples that with information about the disease dynamics in the underlying population,” Dr. Stein explained. “Each time the patient takes a new test, the algorithm updates its predictions.”
From rocket science to medicine. Introduced in the 1960s, the Kalman filter was originally designed to strip unwanted statistical noise out of dynamic data streams, notably those necessary for complex navigational tasks such as helping engineers to launch satellites.2 More recently, researchers have explored the use of Kalman filtering to help monitor chronic conditions such as diabetes and prostate cancer.3,4
For their glaucoma study, Dr. Stein and colleagues evaluated longitudinal data collected from 571 patients in the Collaborative Initial Glaucoma Treatment Study (CIGTS) and the Advanced Glaucoma Intervention Study (AGIS). After first performing logistic regression to eliminate confounding variables, they applied the Kalman-based algorithm and compared its forecasts to actual test values subsequently observed in the patients.
Promising results. The researchers found that the algorithm accurately predicted OAG stability or progression in individual patients and improved the overall efficiency of identifying progression by 29 percent compared with testing at fixed intervals of 1.5 and 2 years (p < .0001 for all comparisons). The algorithm also reduced time to diagnosis of progression by 57 percent compared with fixed annual monitoring, from 3 months to 1.29 months (p = .02).
More studies ahead. Dr. Stein said that their multidisciplinary group hopes by the end of 2014 to seek federal funding for a prospective study of the TNT forecasting algorithm in real-world OAG patients.
—Linda Roach
___________________________
1 Schell GJ et al. Ophthalmology. 2014 Apr 7. doi:10.1016/j.ophtha.2014.02.021.
2 Cipra B. SIAM News. 1993;26:5. www.cs.unc.edu/~welch/kalman/siam_cipra.html.
3 Kuure-Kinsey M et al. Conf Proc IEEE Eng Med Biol Soc. 2006;1:63-66.
4 Lavieri MS et al. IIE Trans Healthc Syst Eng. 2012;2(1):62-77.
___________________________
Dr. Stein holds a provisional patent on the intellectual property described in the study.
Comprehensive Update
Endophthalmitis Trends
Monitoring the causative pathogens of endophthalmitis and their antibiotic sensitivities is important for detecting trends that may change management approaches or reaffirm current practice. Researchers at The New York Eye and Ear Infirmary (NYEE) of Mount Sinai in New York investigated trends at their institution over the past 25 years and found no major changes in the organisms causing endophthalmitis. Coagulase-negative staphylococcus remained the most prevalent pathogen (about 40 percent), followed by Streptococcus viridans species (12 percent) and Staphylococcus aureus (11 percent). Gram-negative organisms accounted for about 10 percent of isolates, and fungi for 4.6 percent.1
 |
|
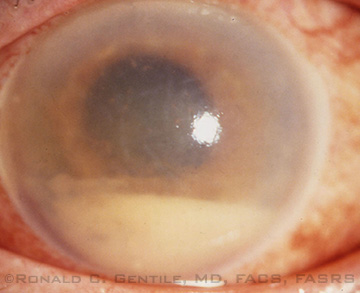
ENDOPHTHALMITIS. Affected eye with cloudy cornea and hypopyon.
|
Changing patterns of resistance. Microbial resistance to many of the betalactam antibiotics—including first-, second-, and third-generation cephalosporins—is continuing to increase. Specifically, the study showed increasing resistance to cefazolin (up to 57 percent resistant). This trend was not detected for cefuroxime, even though up to 25 percent of isolates were resistant to it. “This may be somewhat reassuring to those using cefuroxime prophylactically in cataract surgery, but it still needs to be closely monitored,” said study author Ronald C. Gentile, MD, FACS, FASRS, professor of ophthalmology and Surgeon Director at NYEE.
Methicillin-resistant Staphylococcus aureus (MRSA) isolates also increased from 18 percent in the late 1980s to just over 50 percent this past decade. Although this is concerning, the good news is that vancomycin and ceftazidime continue to be excellent empiric antibiotics for treating endophthalmitis in New York City. More than 90 percent of gram-negative isolates were susceptible to ceftazidime, and over 99 percent of gram-positive isolates were susceptible to vancomycin.
Gains and losses. “One very interesting aspect of our study is that as resistance rates to beta-lactam antibiotics increased, there was a significant decrease in resistance rates to aminoglycosides,” said Dr. Gentile. Gentamicin-resistant strains decreased from 42 percent to 6 percent. This was most likely the result of continued use of beta-lactam antibiotics in a setting of reduced use of aminoglycosides. “Just as selective pressure on bacteria exposed to certain antibiotics causes emergence of resistant strains, the opposite is also true,” said Dr. Gentile.
—Gabrielle Weiner
___________________________
1 Gentile RC et al. Ophthalmology. 2014 Apr 2. doi: 10.1016/j.ophtha.2014.02.001.
___________________________
Dr. Gentile reports no related financial interests.
Cataract Study
SSRIs and Cataract Risk
The growing use of the antidepressants known as selective serotonin reuptake inhibitors (SSRIs) may be increasing the need for cataract surgery, reported a team of Mayo Clinic researchers.1
Though this study demonstrates association, not causation, the finding is important given that antidepressants, mainly SSRIs, are the third most commonly prescribed drug class in the United States, said Jay C. Erie, MD, professor of ophthalmology at Mayo Clinic in Rochester, Minn. SSRI use has more than doubled in the last decade, roughly corresponding to the study years, 2004 through 2011.
More cataract surgery in SSRI group. This case-control study included 6,024 patients aged 50 and older who had undergone cataract surgery; they were matched by age and gender to an equal number of controls without cataract surgery. SSRI use was associated with an increased risk of cataract surgery: 17 percent of the surgery cohort had taken SSRIs for one or more years, compared with 13 percent of controls (odds ratio, 1.36; 95 percent confidence interval, 1.23-1.51; p < .001).
The association held after adjustment for diabetes and oral corticosteroid use, both of which have been linked to cataract risk. The study could not control for smoking or secondhand smoke, potential confounders that need to be evaluated in future studies, Dr. Erie said.
No call to change drugs yet. The SSRIs citalopram and sertraline were significantly associated with cataract surgery, while other SSRIs were not. Because the small sample size for other medications could account for that difference, the findings weren’t strong enough to prompt prescription recommendations, Dr. Erie said, emphasizing that there is no reason to avoid or discontinue SSRIs, which are beneficial to a wide range of people. But, he noted, “changing prescription patterns for SSRIs may be increasing the need for cataract surgery.”
—Miriam Karmel
___________________________
1 Erie JC et al. Am J Ophthalmol. 2014 Mar 14. doi: 10.1016/j.ajo.2014.03.006.
___________________________
Dr. Erie reports no related financial interests.