Download PDF
The promise and pitfalls of biologic therapy.
Monoclonal antibodies, soluble receptors, cytokines, and more—biologics offer an abundant array of therapeutic agents, bioengineered by recombinant DNA technology and focused on a molecular understanding of disease pathogenesis. Developed initially to treat systemic conditions such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis, biologic therapies have also often improved the ocular inflammation of noninfectious uveitides linked with these conditions, and they have subsequently been studied more specifically for the treatment of uveitis.1
“From a bench scientist’s point of view, the biologics are very interesting in terms of how they work immunologically,” said Jennifer E. Thorne, MD, PhD, at the Wilmer Eye Institute in Baltimore. “And from a clinician’s point of view, we’re making headway in getting some of these drugs studied and approved by the U.S. Food and Drug Administration [FDA] for uveitis, although it’s slow going.”
What Biologics Have to Offer
For many years, corticosteroids have been the mainstay of treatment for noninfectious uveitis. But now, biologic therapy, which targets specific cytokines or receptors involved in inflammation, “is beginning to revolutionize the treatment of uveitis,” said H. Nida Sen, MD, MHS, at the National Eye Institute in Bethesda, Maryland. “Biologics tend to be more effective, better tolerated by the patient, and easier to comply with than medication taken by mouth. Administration commonly involves subcutaneous injection or intravenous infusion every other week or less frequently.”
Still, biologics are not a panacea, said James P. Dunn, MD, at Wills Eye Hospital in Philadelphia. Response can be inconsistent between patients, and there are concerns about the potential for long-term toxicity. Moreover, lack of insurance coverage remains a major barrier.
Tested, yet untested. Ophthalmologists gained anecdotal knowledge about biologics and started testing them following their use in rheumatology, gastroenterology, and dermatology, said Dr. Dunn. Still, ophthalmology’s direct experience with biologics for uveitis is limited to about 15 years, said Justine R. Smith, FRANZCO, PhD, at Flinders University in Adelaide, South Australia. “If I had uveitis and my disease responded well to methotrexate, would I want to be on a biologic?” she asked. “No, I would want to take methotrexate, which we’ve had much longer. We know its side effect profile really well, and it’s much less expensive.”
Homing in on the target. “Biologics offer an advantage of more targeted therapy,” said Dr. Thorne, “but uveitis encompasses at least 30 different syndromes, and many of these diseases are undifferentiated with no known definable cause or specific mechanism.”
Progress is being made in understanding the inflammatory pathways involved in uveitis, said Dr. Dunn. “But we still tend to think of this as a linear process that can be completely short-circuited by 1 drug. However, sometimes we block 1 pathway and nature finds a way around it, following another inflammatory pathway. We’ve long known this to be true for other conditions such as asthma.”
Quick effects, but not a cure-all. Some biologics have the advantage of working quickly, unlike traditional immunosuppressants, which may require a month or more to take full effect, said Dr. Dunn. “From my experience, though—there aren’t any good studies comparing the 2—I’m not sure the biologics are any more effective than traditional immunosuppressants, which only seem to work in about two-thirds of cases.” However, he added, biologics can be particularly effective for certain conditions, such as Behçet disease, and helpful for those who fail traditional therapy.
Better tolerated? Corticosteroids have been a mainstay of treatment for noninfectious uveitis for some time. However, when corticosteroids are used for many years, there’s real concern about developing an imbalance in the benefit/harm equation, said Richard Lee, MRCOphth, PhD, at the National Institute for Health Research Moorfields Biomedical Research Centre in London. “With a plethora of associated harms—including diabetes mellitus and high blood pressure—prednisone is the bogeyman of the physician community.”
Physicians must weigh the side effects of long-term oral steroid therapy with the risks of biologics, mainly infection, Dr. Dunn added. Despite their potential for side effects, he said, the immunosuppressants have a better long-term profile than prednisone at doses of 10 mg a day.
One could argue, however, that side effects of biologics may be worse in some patients with uveitis than it is for patients with systemic diseases, said Dr. Smith. “That may be because you may block too much of a substance that is also necessary for normal immune function.” In contrast, she said, “Patients with systemic inflammatory disease may retain enough [of the targeted substance] after treatment because they started out with higher levels.”
 |
|
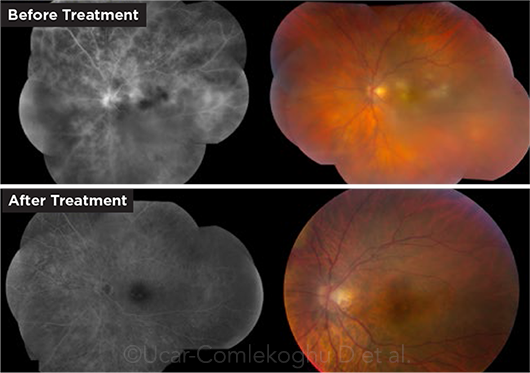
BEHÇET. This 30-year-old patient had macular retinitis and vasculitis (top images). He responded favorably to infliximab infusions, and resolution of both his retinitis and vascular leakage was noted (bottom images).
|
TNF-Blockers
Of the different classes of systemic biologics, tumor necrosis factor (TNF)–blockers are the most commonly studied and used for uveitis, said Dr. Sen. “Some of these block circulating TNF, while others block both circulating and membrane-bound TNF.”
TNF-blockers are manufactured using molecular biological techniques and are typically monoclonal antibodies, which means they are very specific in the proteins they target in the immune system, Dr. Lee said. “By targeting TNF, they are essentially neutralizing this very proinflammatory mediator of the immune cascade.”
In other diseases. TNF-blockers have completely revolutionized the field of rheumatology, said Dr. Lee. “People with rheumatoid arthritis have a very different future because of these drugs. They have also been a game-changer in certain inflammatory bowel diseases. In the United Kingdom, the spending on the TNF-blockers by our National Health Service is higher than for any other class of drugs—across the board for all disciplines.”
In uveitis. The basic science underpinning TNF-blockers’ use for uveitis is fairly strong, said Dr. Lee. “Using a preclinical mouse, researchers have shown that cells in the inflamed retina—retinal microglial cells and infiltrating macrophages—produce TNF and [have demonstrated] how neutralizing it modifies the disease.”
TNF has been well established as a factor in uveitis, added Dr. Dunn. “It’s increased in the eye and serum of patients with certain types of noninfectious intermediate and posterior uveitis and panuveitis, and it decreases in patients that have control of their disease.”
Infliximab and adalimumab. In July of 2016, adalimumab (Humira) became the first and only biologic to receive FDA approval for uveitis. “As its trade name suggests, it is made from human proteins,” said Dr. Lee. “The more human the biologic, the less likely your immune system will recognize it as foreign.”
Infliximab (Remicade) is another TNF-blocker more commonly used for uveitis. It is an example of an engineered monoclonal antibody containing foreign proteins from more than one species, a chimera between a human and mouse protein.
Other options. Other TNF-blockers such as golimumab (Simponi) and certolizumab (Cimzia) have been used much less commonly, said Dr. Sen. “They aren’t necessarily less effective. We just don’t have enough data about them. Doctors can consider switching patients to one of these drugs if they have problems with adalimumab or infliximab.”
 |
|
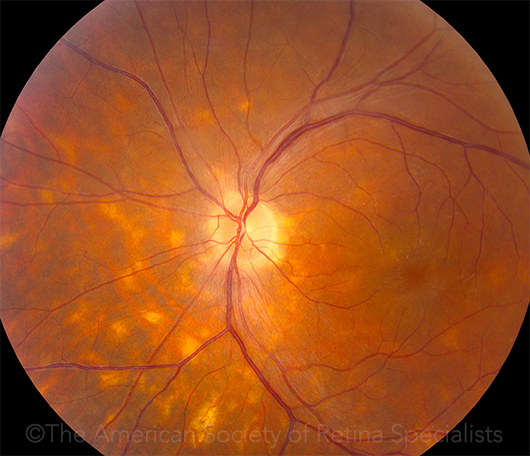
BIRDSHOT. This 42-year-old patient had birdshot chorioretinopathy and cystoid macular edema. This image was originally published in the ASRS Retina Image Bank. Pauline T. Merrill, MD, and John Pollack. BSC CME OS. Retina Image Bank. 2012; Image Number 2098. © The American Society of Retina Specialists.
|
Treating Patients
Here are some guidelines on the use of biologics, though many questions and caveats remain.
Standard approach. “As a rule,” said Dr. Dunn, “many of us still start with steroids, add a traditional immunosuppressant such as an antimetabolite, often mycophenolate mofetil, or a T-cell inhibitor, such as tacrolimus or cyclosporine A. But rather than going to cytotoxic agents as the next step or combining the T-cell inhibitor with an antimetabolite, we’ll try a biologic if it is feasible from the patient’s point of view.”
Care should be individualized. Physicians need to consider what is best for each patient, said Dr. Dunn. For some patients, for example, avoiding systemic side effects may be critical, and systemic therapy may not be the best option; regional corticosteroids might be the better choice. “You need to mix and match options with the patient’s profile. A patient with high blood pressure may not be a good candidate for a T-cell inhibitor. A long-term drinker is not a good candidate for an antimetabolite. This is particularly true for methotrexate because of its potential for added liver damage. And a patient with advanced congestive heart failure is not a good candidate for a TNF-blocker.”
Collaboration is critical. Because the standard care for this type of disease is systemic treatment, physicians need special experience and training to be competent in the use of these drugs, Dr. Lee cautioned. Ophthalmologists, especially those who are not specifically trained in uveitis, will want to co-manage these patients with an internist—often a rheumatologist, immunologist, or gastroenterologist—who has expertise in the use of biologics, Dr. Smith added. This includes communication during pretreatment assessments and, later, during decisions on medication type or dose and the monitoring of side effects and flares.
Nuances with use. Additional treatment nuances that have emerged are as follows:
Earlier is better. “Even though we don’t have many clinical trials in uveitis comparing different types of treatments head to head,” said Dr. Sen, “we do have good retrospective data.” As in rheumatology, the consensus among ophthalmology experts is that early treatment with TNF-blockers is the way to go, said Dr. Lee. “It’s important to intervene before there is irreversible tissue damage inside the eye. The danger of this, however, is you don’t know who needs this high level of treatment. Are you overtreating?”
Staying power is uncertain. Sometimes TNF-blockers are very effective initially but ultimately wear off, said Dr. Lee. “We don’t have enough data yet for uveitis, but the expectation is that the effects will not be sustained for a good proportion of uveitis patients.” Particularly in the case of chimeric drugs like infliximab, he explained, the drugs may wear off because the patient’s immune system recognizes the antibody as foreign.
Doses may need to be finessed. In some cases, a patient may not respond due to a problem with dosing, said Dr. Dunn, and it’s helpful to coordinate with an internist who is monitoring the infusions.
“The standard regimen for treating nonocular diseases such as rheumatoid arthritis with infliximab is to administer 3-5 mg per kg every 8 weeks as a maintenance therapy,” said Dr. Dunn. “It is the experience of most uveitis specialists that eye disease requires higher doses per infusion at shorter intervals. Sometimes we may go up to as much as 10 mg per kg every 4-6 weeks. Adalimumab is given every 2 weeks, but that may be insufficient in some cases, and it may work better if you can get it reapproved at weekly injections, although this is not the FDA-approved labeling.”
When TNF-Blockers Fail
To minimize costs and side effects, uveitis specialists will typically attempt to treat for a period of 2-3 years and then stop the biologics, said Dr. Thorne. “But data telling you how long to treat are limited, because many of these drugs haven’t been around long enough.”
What about treatment failure? In the Visual 1 study (see “Research Update”), treatment failure was 24 weeks in the adalimumab group and 13 weeks in the placebo group. In the Visual 2 study of patients with controlled uveitis, treatment failure occurred in 55% of patients in the placebo group and in 39% of those taking adalimumab, said Dr. Sen. “This is a significant advance, but there’s still a lot of room for improvement.”
When is a flare-up a failure? It’s true that these are not necessarily perfect drugs for everyone, said Dr. Smith. Some patients will not respond; others will have severe side effects. “But ask yourself whether it is truly a failure. Take the example of a patient with Behçet who was previously having flare-ups 5 times a year and has not had one for 3 years. If this patient experiences just 1 flare-up, is this really a failure?” Is a wholesale change necessary, she asked, or could you tide the patient over with the addition of local corticosteroids, for example?
Changes to the biologic regimen. “If it is a particularly aggressive disease, you might alter the regimen, giving the drug more often,” said Dr. Smith. If you’ve exhausted all opportunities of conventional immunosuppressant therapy, and a failure is due to lack of efficacy (or incomplete efficacy or initial response and then failure), said Dr. Dunn, changing from one TNF-blocker to another might work. “However, if the patient has intolerable side effects, it probably doesn’t make sense to try another drug within the same category.”
Combining drugs. Clinicians usually do not combine 2 biologics, due to a higher risk of infection. “With this type of combination, 1 trial found a significant increase in infection without a significant increase in effectiveness,” said Dr. Sen. But combining biologics with nonbiologics is commonly done, she said. Thus, a patient might be on a TNF-blocker and an antimetabolite (for example, methotrexate or mycophenolate mofetil) or a T-cell inhibitor (such as cyclosporine or tacrolimus). Rarely, drugs such as rituximab (Rituxin) and alkylating agents such as cyclophosphamide have also been combined. TNF inhibitors typically are not combined with other biologics.
What is the advantage of combining biologics along with mycophenolate mofetil, for example? Is there an additive effect, where both drugs control the abnormal immune response? Or is mycophenolate mofetil preventing the production of antibodies by the host immune system, which neutralizes the drug? It is likely both, said Dr. Lee.
 |
|
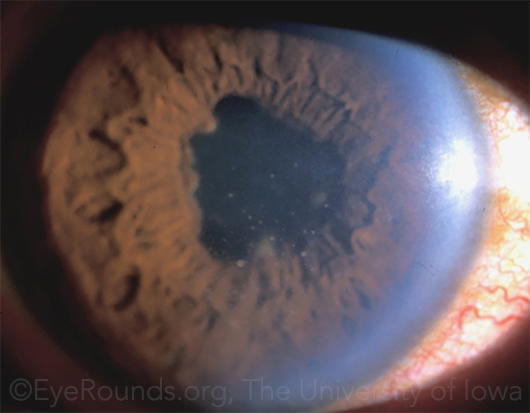
CAUTION. TNF-blockers can reactivate infection in patients with certain diseases, including tuberculosis and hepatitis. Thus, patients such as this one (whose granulomatous uveitis was related to tuberculosis) must be treated first for the underlying disease, and there must be no evidence of active infection.
|
What About Side Effects?
Although they can cause harm, most patients can tolerate the TNF-blockers, said Dr. Lee.
Infection an issue. An increased risk of infection, requiring antibiotics, occurs most commonly, said Dr. Thorne. Seen in AIDS patients, very severe infections such as progressive multifocal leukoencephalopathy can occur, albeit rarely.
“Patients are at risk for reactivation of infections such as tuberculosis, histoplasmosis, hepatitis, and fungal infection,” said Dr. Dunn. Patients with infections like these can still go on a TNF-blocker, he said, but they must first be treated for the infection, and there must be no evidence of active disease.
Screening needed. In addition to interferon gamma release assays to spot previous TB exposure, said Dr. Lee, patients may require brain imaging to rule out the demyelinating hot spots of multiple sclerosis, which may worsen with use of TNF-blockers. “Any patient with demyelinating disease should not use a TNF-blocker,” added Dr. Dunn. “Although intermediate uveitis may be a harbinger of multiple sclerosis, adalimumab may be used if central nervous system demyelination is first excluded.”
Other side effects. Other less common side effects of biologics include malignancies, particularly for nonmelanoma skin cancers, said Dr. Dunn. Patients may also develop lupus-type disease (this is more common if they have developed antibodies to the protein) and sensitivity to the drugs, including skin reactions or more severe systemic allergic reactions. He added, “TNF antagonists are weak negative inotropic agents, which change the force of the heart’s contractions. This means they shouldn’t be used in patients with advanced congestive heart failure.”
Additional concerns. Finally, TNF-blockers should be used judiciously or not at all if a patient is pregnant or nursing, said Dr. Sen, even though the drugs are considered pregnancy risk category B (meaning no evidence of risk in humans). “If patients must use them, they are safer than methotrexate, for example, which is a pregnancy risk Category X drug” and thus should not be used during pregnancy. Dr. Sen also counsels patients against using live vaccines while they are being treated with immunosuppressants.
Other Biologic Targets
“At the moment, we are focused on the cytokines and the receptors,” said Dr. Smith, “but there are many potential targets for biologics in the eye, including adhesion molecules, the complement system, chemokines, oxidative stress, growth factors, the inflammasomes, and the ubiquitin proteasome systems. The field has the potential to really blossom over the next 10 or 20 years.”
Beyond infliximab and adalimumab, here are a few of the drugs that have been tried or are in the pipeline for patients with uveitis:
Rituximab. Injected into the eye for patients with vitreoretinal lymphoma, rituximab is also being given systemically to treat patients with orbital inflammatory disease and scleritis, said Dr. Smith. “More recently it has been used in patients with uveitis,” including kids with uveitis associated with juvenile idiopathic arthritis (JIA). Rituximab targets B cells, but its specific mechanism of action in uveitis is poorly understood, said Dr. Lee.
Interleukin blockers. Interleukin-6 receptor (IL-6) blockers are a reasonable alternative for uveitis when other drugs fail, said Dr. Lee, although the data in the literature are not of the highest level. “IL-6 appears to be good for restoring the blood-ocular barrier and for getting rid of macular edema, but it is less effective at eradicating other features of inflammation.” Although its use is supported mainly by case reports and small case series, tocilizumab (Actemra) is an IL-6 blocker that shows some promise, said Dr. Thorne, and it is currently being evaluated in the STOP-UVEITIS multicenter clinical trial.
The IL-1 blocker gevokizumab initially looked encouraging but failed to reach primary endpoints in large clinical trials, said Dr. Smith. Daclizumab (Zinbryta), which targets the IL-2 receptor and showed promising results in small clinical trials in uveitis patients, was taken off the market temporarily because of poor market demand, but it is now available again off label, Dr. Sen said.
___________________________
1 Pasadhika S, Rosenbaum JT. Biologics. 2014;8:67-81.
Research Update
Clinicians who are searching for additional guidance on the use of biologics will come up against the fact that researchers have conducted few randomized biologic trials for noninfectious uveitis.
Here are some research challenges to consider:
Too few patients. Given the low prevalence and variety of types of uveitis, it is extremely hard to achieve meaningful statistical outcomes when conducting biologic trials, Dr. Dunn noted. And while the research that has been conducted in ophthalmology has given clinicians a sense of which biologics are likely to be effective, other fields—where there are larger numbers of patients—will continue to drive drug development, Dr. Smith said.
Same tissue, different diagnosis. Classified anatomically, a group of patients with posterior uveitis might all be lumped together in a trial, said Dr. Smith, even though one might have birdshot chorioretinopathy, another serpiginous choroidopathy, and a couple of others idiopathic retinal vasculitis. “This would be like investigating patients with any type of inflammatory arthritis of the joints—whether rheumatoid arthritis, ankylosing spondylitis, or sarcoidosis—and grouping their results together,” she said.
Measuring outcomes. It’s challenging to identify the appropriate outcome measure for different diseases that can be applied across the board, said Dr. Smith, especially since it may not be best for a specific type of disease. “For this reason, some trials have used a composite endpoint, which might include visual acuity, resolution of macular edema, and ability to wean off corticosteroids.”
Unanswered questions. An expert panel paper published in late 2014 culled the evidence on using TNF-blockers for ocular inflammatory disease to that date, analyzing more than 400 publications from the previous 15 years.1 “Among the panel’s recommendations were that a TNF-blocker like infliximab could be used as a first-line therapy for Behçet uveitis,” said Dr. Thorne. The data also supported second-line use in chronic uveitis associated with JIA, she said.
The recommendations provide limited guidelines for when to consider biologics for other specific types of uveitis or scleritis, said Dr. Sen. “Most importantly, there is no guideline or biologic marker to indicate when it is safe to stop biologics. This still needs to be answered.”
Specific Trials
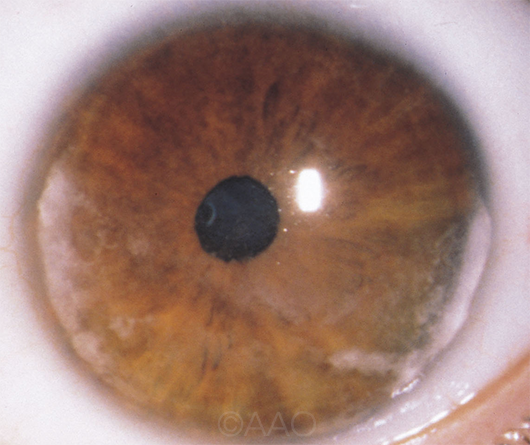
JIA. In the first randomized controlled study of adalimumab in children with JIA-associated uveitis, adalimumab plus methotrexate controlled inflammation better than methotrexate alone.
Despite these challenges, the following trials have been completed:
Visual 1 and 2. These trials found that adalimumab lowered the risk of uveitic flare or loss of visual acuity upon withdrawal of corticosteroids in patients with inactive uveitis controlled by systemic corticosteroids.2,3
“These studies showed unequivocal benefit leading to FDA and European Medicine Agency licensing of adalimumab, which is a real milestone,” said Dr. Lee. Following completion of these trials and approval of adalimumab, said Dr. Thorne, it is possible biologics will more frequently be used as second-line therapy for intermediate and posterior uveitis and panuveitis in adults, where they showed efficacy.
SYCAMORE. The first randomized controlled trial to study children treated with adalimumab combined with methotrexate for JIA-associated uveitis was recently completed in the United Kingdom.4 Researchers found that the combination was significantly more effective at controlling inflammation and resulted in fewer treatment failures than did methotrexate alone. “The trial was stopped early because the benefits were so profound,” Dr. Lee commented.
And the study points to a way forward in terms of methodology, Dr. Lee pointed out: By focusing on a single type of uveitis, as the researchers did in this instance, “we might be able to really separate the signal from the noise.”
___________________________
1 Levy-Clarke G et al. Ophthalmology. 2014;121(3):785-796.
2 Jaffe GJ et al. N Engl J Med. 2016;375(10):932-943.
3 Nguyen QD et al. Lancet. 2016;388(10050):1183-1192.
4 Ramanan AV et al. N Engl J Med. 2017;376(17):1637-1646.
|
Meet the Experts
James P. Dunn, MD Director of the Uveitis Unit at Wills Eye Hospital in Philadelphia. Relevant financial disclosures: AbbVie: C,L.
Richard Lee, MRCOphth, PhD Lead for Experimental Medicine, Inflammation, and Immunotherapy Theme at the NIHR Moorfields Biomedical Research Centre and deputy director at the NIHR Moorfields Clinical Research Facility in London; consultant senior lecturer at the University of Bristol and Bristol Eye Hospital in Bristol, U.K.; and senior visiting clinician scientist at the National Eye Institute in Bethesda, Md. Relevant financial disclosures: None.
H. Nida Sen, MD, MHS Director of the Uveitis and Ocular Immunology Fellowship Program at the National Eye Institute in Bethesda, Md. Relevant financial disclosures: None.
Justine R. Smith, FRANZCO, PhD Matthew Flinders Distinguished Professor of Eye & Vision Health at Flinders University in Adelaide, South Australia. Relevant financial disclosures: AbbVie: C.
Jennifer E. Thorne, MD, PhD Chief of the Division of Ocular Immunology, the Cross Family Professor of Ophthalmology, and professor of epidemiology at the Wilmer Eye Institute in Baltimore. Relevant financial disclosures: AbbVie: C,S.
For full disclosures and the disclosure key, see below.
|