Download PDF
A variation on intravitreal therapy, in which treatment intervals are first extended to 12 weeks and then injections are stopped altogether, can preserve the visual acuity (VA) of patients with wet age-related macular degeneration (AMD) even if their choroidal neovascularization (CNV) recurs, a study by California retina specialists has found.
Their retrospective analysis of outcomes with this “treat-extend-stop” (TES) protocol showed that 37.3% of 385 eyes treated for CNV met the criteria for cessation of therapy. Of these 143 eyes, 70.6% required no further intervention during a mean of 27 months of follow-up. In those that did have a recurrence, 54.8% recovered after retreatment to 20/40 or better, similar to the mean VA in the group when the injections were stopped.1 Patients were able to maintain a 2-line gain in vision (10 letters), even after a recurrence.
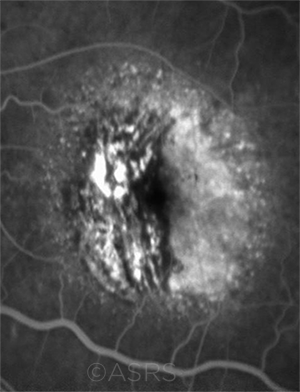 |
ACTIVE. A 30-degree fluorescein angiogram of an 80-year-old patient with wet AMD and active CNV. This image was originally published in the ASRS Retina Image Bank. Carolyn Daley. Neovascular AMD With CNV. Retina Image Bank. 2018; Image Number 27695. © The American Society of Retina Specialists.
|
Rationale. “Originally I did this study just out of clinical curiosity. I wanted to know what percentage of patients had a recurrence of the CNV after we stopped therapy,” said coauthor Sean D. Adrean, MD, who practices in Fullerton, California. “I was relieved to find out that when patients had a recurrence, overall they did not lose vision.”
Dr. Adrean said that, based on the literature and on anecdotal reports from colleagues around the country, the most commonly used treat-and-extend protocol for intravitreal treatment of CNV lengthens the interval between injections to 10-12 weeks and continues this schedule indefinitely. But his analysis of 8 years of outcomes in his group’s practice suggests that many patients with wet AMD could benefit from the TES approach, he said.
“To know that you can actually stop injecting these patients and they can continue to do well, and only about 30% of eyes [experience a recurrence]—well, this means that you can maybe avoid doing unnecessary injections, or at least reduce the number of injections the patient needs,” Dr. Adrean said.
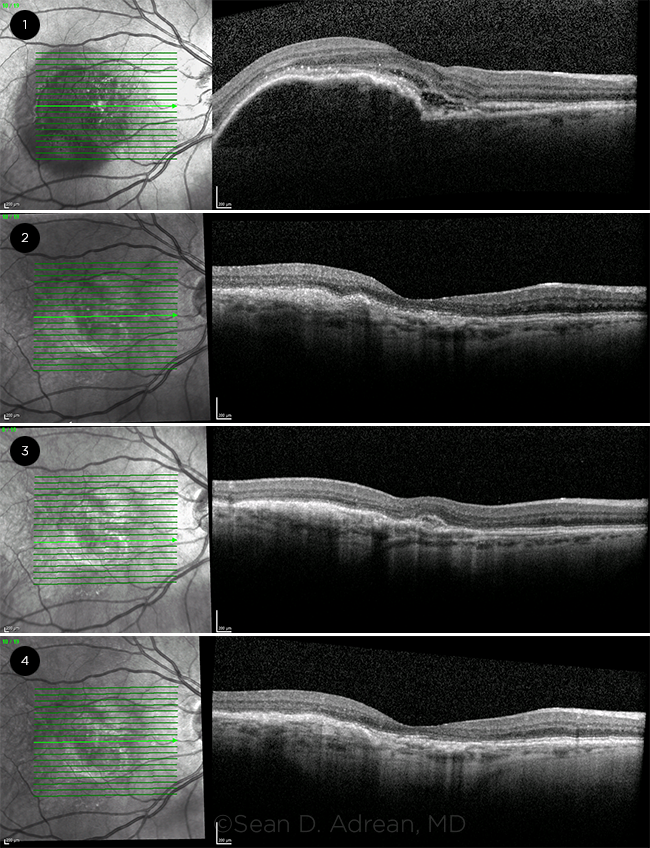 |
CASE EXAMPLE. (Fig. 1) This 78-year-old patient with wet AMD presented with 20/200 visual acuity (VA) in May 2009. (Fig. 2) Fifty-five months later, after he had received 37 bevacizumab injections under the TES protocol, his CNV regressed, and his VA improved to 20/20-. (Fig. 3) Fourteen months later, he presented with increased metamorphopsia and VA of 20/40, and SD-OCT showed an early recurrence of the CNV. (Fig. 4) Once anti-VEGF therapy resumed with several injections, his vision improved to 20/25.
|
TES protocol. The authors described their TES protocol as follows:
- Therapy begins with at least 3 monthly injections of an anti–vascular endothelial growth factor (VEGF) agent, until a “dry” macula is confirmed with spectral-domain optical coherence tomography (SD-OCT).
- If the macula remains free of fluid, the intervals between injections are extended by 1 to 2 weeks between successive visits, until a 12-week time interval is reached.
- If the patient has received at least 7 total injections, and if SD-OCT at 3 successive 12-week visits confirms that CNV has not recurred, the injections are stopped.
- Patients return 1 month later and then successively longer by 2-week intervals until 12 weeks is reached. These patients are then monitored quarterly for signs of recurrence.
Monitoring a must. Because the study documented recurrences in a few TES patients as long as 3 years after the cessation of treatment, ongoing monitoring every 3 months is essential, Dr. Adrean said.
“I’m always very frank with them, and I say there’s a 30% chance that their CNV could come back. I tell them to come back earlier if they have increased distortion or decreased vision, so we can start treatment again if the CNV has recurred,” he said.
—Linda Roach
___________________________
1 Adrean SD et al. Ophthalmology Retina. 2018;2(3):224-229.
___________________________
Relevant financial disclosures—Dr. Adrean: Genentech: C,S; Ohr Pharmaceuticals: C,S; Ophthotech: C,S; Regeneron: C,S; SciFluor Life Sciences: C,S.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Adrean Allergan: C,S; Genentech: C,S; Ohr Pharmaceuticals: C,S; Ophthotech: C,S; Regeneron: C,S; SciFluor Life Sciences: C,S.
Dr. Fieß None.
Dr. Medeiros Alcon: C; Allergan: C; Bausch + Lomb: S; Carl Zeiss: C,S; Heidelberg Engineering: C,S; Merck: S; NIH: S; Novartis: C; Sensimed: S; Topcon: S; Reichert: C,S.
Dr. Whalen None.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review