Download PDF
Researchers have known since the 1970s that subclinical neovascularization could exist in eyes with dry age-related macular degeneration (AMD).1,2 Two decades later, indocyanine green angiography (ICG) showed that, in patients with wet AMD in one eye and dry AMD in the other, these subclinical features were a strong predictor for development of exudative disease in the second eye.3,4 However, monitoring eyes with repeated ICG testing was too invasive and expensive to be practical.
Now, results of a study using swept-source optical coherence tomography angiography (SS–OCT-A) suggest that the technique might enable ophthalmologists to not only identify eyes with these risky subclinical lesions but also quickly begin treatment when symptomatic leakage occurs.5
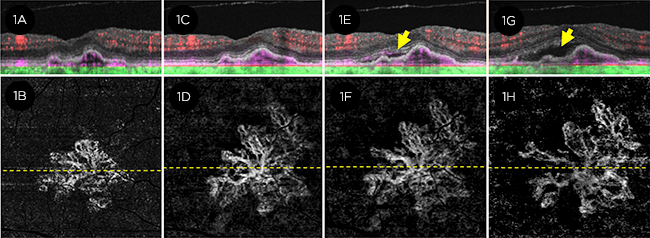 |
CONVERSION. This eye had subclinical type 1 MNV at baseline (1A, 1B) and demonstrated exudative disease during 24 months (1G, 1H) of follow-up. (Top images, OCT B-scan; bottom images, SS–OCT-A. Arrow = increase in subretinal fluid.)
|
Predicting risk. For this prospective study, researchers used SS–OCT-A to monitor the disease status in 160 eyes with intermediate dry AMD or geographic atrophy (GA). The patients had wet AMD in the fellow eye.
The investigators found that, after 1 year of follow-up, exudative disease developed in 21.1% of the eyes with subclinical macular neovascularization (MNV) at baseline. The risk for exudation was 15.2 times greater (95% confidence interval, 4.2 to 55.4) compared with eyes without subclinical MNV, the scientists reported.
“This has enormous predictive value. It provides us with a tool that allows us to identify those patients who are most at risk, so they don’t fall through the cracks,” said coauthor Philip J. Rosenfeld, MD, PhD, at the Bascom Palmer Eye Institute in Miami.
“This finding alone—that we can identify these subclinical lesions long before exudation occurs—provides all the rationale we need to justify the use of this noninvasive, safe, and easily performed technology to survey all our patients with intermediate AMD or geographic atrophy,” Dr. Rosenfeld said.
Additional findings. The researchers also reported the following results:
- Overall, 14.4% of the 160 study eyes showed subclinical MNV at baseline.
- One year after the first observation of subclinical MNV (either at baseline or during follow-up), 24% of these eyes developed exudation.
- Of the eyes without subclinical MNV on their initial angiogram, 5.4% developed it within 1 year. In these eyes, a druse-like elevation of the retinal pigment epithelium (RPE) was identified at the site. “We believe that these RPE elevations were the first sign of type 1 MNV and can serve as harbingers of impending exudation,” the authors wrote.
An essential technology? Dr. Rosenfeld said he views OCT-A as “a requirement for anyone with dry AMD.” If subclinical neovascularization is present, he repeats the procedure at least every 2 months. However, he initiates treatment only if the patient becomes symptomatic, he said.
OCT-A can be used to help ophthalmologists avoid unnecessary intravitreal injections, Dr. Rosenfeld said. “Treat as early as possible, but don’t treat unless there’s exudation. Remember that fluid can come and go in the retina of a patient with dry AMD in the absence of neovascularization, without serious consequences,” he said. “And SS–OCT-A can identify those patients who have fluid in the absence of neovascularization.”
Dr. Rosenfeld said the barriers to greater use of swept-source OCT to monitor patients with dry AMD are financial: Specifically, the device is double the price of earlier OCT devices.
But the technique’s potential to help manage dry AMD patients is enormous, he said. “This strategy will save more vision in the long run than we’ve been able to accomplish” with intravitreal injections, he said. “We’ll be able to treat as soon as symptomatic exudation occurs. Home monitoring will be important as well, but now we can select those patients at highest risk even when they don’t have the typical high-risk fundus findings on exam.”
—Linda Roach
___________________________
1 Sarks SH. Br J Ophthalmol. 1973;57(12):951-965.
2 Green WR, Key SN III. Trans Am Ophthalmol Soc. 1977;75:180-254.
3 Schneider U et al. Int Ophthalmol. 1997;21(2):79-85.
4 Hanutsaha P et al. Ophthalmology. 1998;105(9):1632-1636.
5 de Oliveira Dias JR et al. Ophthalmology. Published online Sep 27, 2017.
___________________________
Relevant financial disclosures—Dr. Rosenfeld: Apellis: C,O; Carl Zeiss Meditec: C; Genentech: C,S.
For full disclosures and disclosure key, see below.
More from this month’s News in Review