By Linda Roach, Contributing Writer, interviewing Louis B.Cantor, MD, Richard A. Lewis, MD, and David Southwell
Download PDF
New approaches for treatment of uncontrolled glaucoma are in the drug development pipeline. The 2015 Ophthalmology Innovation Summit (OIS), held in Las Vegas before AAO 2015, provided an overview of 2 new glaucoma medications—Rhopressa and trabodenoson.
Glaucoma Target: The Trabecular Meshwork
“Glaucoma with elevated intraocular pressure [IOP] is primarily a disease of the outflow system through the trabecular meshwork [TM], but over the last 2 decades we’ve moved away from enhancing trabecular flow, as our newer drugs worked through different mechanisms and our older medications, such as pilocarpine, caused a number of local ocular side effects,” said Louis B. Cantor, MD, of Indiana University.
Today’s most commonly used drugs reduce IOP by enhancing uveoscleral outflow or suppressing aqueous production, Dr. Cantor said. Not since the advent of prostaglandin analogues nearly 2 decades ago has there been a topical medication for glaucoma that is based on a completely new mechanism of action, he added.
So glaucoma specialists are eagerly anticipating the possibility that this drought might end this year, with the expected submission to the FDA of Rhopressa, the first compound aimed at making the TM more permeable.
Meanwhile, the developer of a second TM-targeted drug, trabodenoson—which works via a different molecular mechanism—hopes to bring the drug to the FDA for consideration in 2018.
“These new drugs appear to affect trabecular outflow, and that takes us back to a therapeutic target that’s really the source of the disease,” Dr. Cantor said.
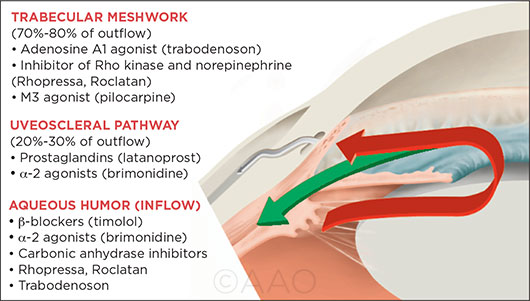 |
|
3 DRUG TARGETS. Current glaucoma drugs reduce elevated IOP by decreasing aqueous humor production and increasing uveoscleral outflow (green arrow). Rhopressa and trabodenoson represent new classes of drugs, which aim to enhance natural flow through the TM (red arrow) without the side effects of pilocarpine.
|
AR-13324 (Rhopressa)
This topical inhibitor of the Rho kinase (ROCK) and norepinephrine transport (NET) pathways completed a phase 3 noninferiority trial in September 2015. Aerie Pharmaceuticals, developer of the drug, plans to submit the results (noninferiority to twice-daily timolol) to the FDA in mid-2016, seeking an indication of once-daily use to reduce IOP when the baseline pressure is under 25 mm Hg, said Vicente Anido Jr., PhD, Aerie’s CEO and chairman, speaking at OIS.
However, even if the FDA agrees to make Rhopressa the first FDA-approved drug to target the TM, the results of another Aerie clinical trial suggest that its greater impact on the battle against IOP might come from combining it with a prostaglandin analogue.
Combination therapy? In that phase 2b trial in 223 glaucoma patients, Aerie’s combined formulation of Rhopressa and latanoprost, called Roclatan (PG324), was compared with monotherapy with Roclatan’s component drugs. Levels of IOP reduction were equivalent between the Rhopressa-only and latanoprost-only groups at all measured time points over a 30-day period. But mean IOP was 2 to 3 mm Hg lower in the Roclatan eyes than in those other 2 groups at all time points (p < .001), Dr. Anido reported at OIS. A phase 3 study with a similar design is under way, with results expected late this year.
The mechanism: ROCK/NET inhibition. Rhopressa appears to work by inhibiting receptors in the ROCK enzymatic pathway, which has been linked in animal studies to TM fibrosis, and by reducing NET activity, which decreases aqueous production, Dr. Cantor said.
“ROCK receptor [inhibitors] have been talked about at meetings for a number of years, but none of them really captured much attention because the efficacy was marginal,” said glaucoma specialist Richard A. Lewis, MD, Aerie’s chief medical officer. “But this particular drug has caused a lot of excitement because we have evidence to believe that Rhopressa is actually directed at the trabecular meshwork, and because it has worked so well in phase 2 trials. It’s also exciting because it can be used in conjunction with a prostaglandin analogue as an additive drug.”
A surprise. “When you dissect the results from the Rhopressa studies, you find that you get a similar effect at lower mean levels of IOP as you do with higher levels,” Dr. Cantor said. “And that’s not typical. Other drugs to date work better at higher pressures.”
Animal studies suggest that this might be because Rhopressa adds a new wrinkle to the IOP story. That is, apart from affecting the TM, it might also be reducing episcleral venous pressure, which normally contributes about half of total IOP in healthy eyes, Dr. Lewis said. Further studies are being done to investigate this hypothesis, he said.
If Rhopressa does prove to reduce episcleral venous pressure, new therapeutic possibilities might follow, Dr. Cantor said. “Some of this still has to be borne out in the clinical trials, but it opens up the question of whether we potentially have a drug that will be more effective, for example, in patients with normal-pressure glaucoma, or in patients with advanced glaucoma that may be progressing at lower pressures,” he said.
Trabodenoson
Trabodenoson works via a different molecular mechanism and is further than Rhopressa from having FDA-ready trial results. It is aimed at a different patient population: those with more significantly elevated IOP, 24 mm Hg or higher. Developer Inotek hopes to apply for U.S. marketing approval for trabodenoson by 2018. “We’re less focused on getting the drug onto the market as quickly as we can than we are at getting the right data so that we ultimately have the right label,” said Inotek president and CEO David Southwell. “We’re not doing our phase 3 studies in parallel; we’re doing them in sequence so that we can really learn about the drug.”
Mode of action. Trabodenoson is an adenosine A1 receptor agonist that apparently increases outflow by up-regulating TM cells’ production of matrix metalloproteinases. In normal eyes, these “housekeeping” molecules clear accumulated debris from the meshwork, Dr. Cantor said.
“We know that in glaucoma, one of the earliest changes in the trabecular meshwork is a buildup of proteinaceous material, called glycosaminoglycans, which sort of ‘gums up’ the meshwork and is associated with a decline in the outflow facility,” he said. “So if you can reverse that in the trabecular meshwork, it will drain better.”
Preliminary outcomes. Phase 2 randomized controlled trials of trabodenoson have produced encouraging results, and a larger phase 3 trial to determine efficacy and safety of the monotherapy began last year. The company is also looking at a combined formulation of the new drug with latanoprost, Mr. Southwell said at OIS.
In a study of trabodenoson monotherapy (patients off all other glaucoma medication), reduction from diurnal baseline on day 1 ranged from 3.5 to 5.0 mm Hg, and reduction from pretreatment study baseline at 8 a.m. on day 1 ranged from 4.0 to 7.0 mm Hg. When the drug was added to latanoprost (in patients already on latanoprost), reduction from diurnal baseline on day 1 was 4.3 mm Hg, and reduction from pretreatment study baseline at 8 a.m. on day 1 was 5.8 mm Hg.
Greater effect over time. The investigators also found that IOP continued to fall between the 2- and 4-week exams in the highest-dose monotherapy group (500 μg), reaching a mean reduction of 6.5 mm Hg on day 28 (p = .016 for 2-week difference).
An explanation for the drug’s increasing efficacy may be that the removal of proteinaceous material takes time, Dr. Cantor said. “Presently, it is not known how long it will require to reach the final steady state,” he said.
Both Drugs: Side Effects
Hyperemia was was the most common adverse effect observed with Rhopressa. In once-daily dosing, hyperemia was increased in 35% of Rhopressa patients; it was scored as mild for 83% of these patients, Dr. Anido reported.
In contrast, a handful of patients had mild, transient hyperemia during the first week of trabodenoson monotherapy, which was absent at later exams, Mr. Southwell reported.
___________________________
Dr. Cantor is department chair and professor of ophthalmology, director of glaucoma service, and the Jay C. and Lucile L. Kahn Professor of Glaucoma Research and Education, at Indiana University, in Indianapolis. Relevant financial disclosures: Aerie: C; Allergan: S; Bausch + Lomb: S; Eli Lilly: travel; Innfocus: S; Inotek, S; Mati: O; Mobius: S.
Dr. Lewis practices at Sacramento (Calif.) Eye Associates and is chief medical officer for Aerie Pharmaceuticals, Irvine, Calif. Relevant financial disclosures: Aerie: E.
NOTE: Biographical information and financial disclosures are not provided for corporate speakers at OIS—Mr. Anido and Dr. Southwell—as their roles and proprietary interests are self-evident.
See the disclosure key at www.aao.org/eyenet/disclosures.