Download PDF
The allure of turning brown eyes blue is strong for some people—so strong that they sometimes balk at giving up the cosmetic iris implants that are imperiling their vision. And researchers at the University of California, Los Angeles (UCLA) have confirmed that ocular complications related to the devices occur not only before but also many months after explantation.1
“Despite me telling them, ‘Your eye is not doing well, your cell count is down, you’re in pain, your pressure is 30, your eye is all inflamed, you need to get these things out,’ they say, ‘OK, I’ll think about it.’ A year later, they come back, and their eye is totally decompensating. They just don’t want to let the implants go once they get them in their eyes,” said Kevin M. Miller, MD, at UCLA.
Dr. Miller made those observations while he and his coauthors treated implant complications and then studied postexplantation outcomes in 12 people (24 eyes) with brown irides. All wanted blue eyes and sought out the unapproved anterior chamber implants, which were inserted in France, Jordan, Mexico, Panama, and Turkey. (For more, including a discussion of body dysmorphic disorder in these patients, see Practice Perfect in the March issue.)
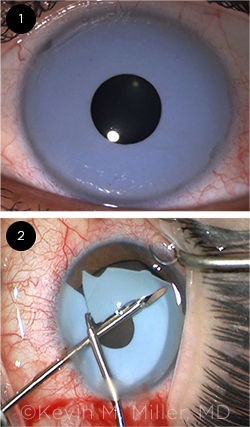 |
RELATIVELY LUCKY. (1) This patient—seen with the cosmetic implant in place—had a low endothelial cell count preoperatively, but he was fortunate in that his eye was otherwise unremarkable. (2) The intraoperative photo shows the segmentation and removal of the implant. During surgery, care must be taken to avoid further damaging the cornea and touching the native iris and crystalline lens.
|
Before explantation. The mean interval from implantation to presentation was 61.7 ± 60 months. Complications at presentation included iris abnormalities (11 eyes, 45.8%), elevated intraocular pressure (eight eyes, 33.3%), corneal edema (six eyes, 25%), intraocular inflammation (five eyes, 20.8%), and cataract (four eyes, 16.7%).
Initial surgical interventions included cataract extraction, corneal transplantation, and glaucoma surgery.
These findings are similar to those from smaller case studies over the past decade, but the UCLA study expands on this literature by showing that ocular complications arise even after the implants are removed, Dr. Miller said.
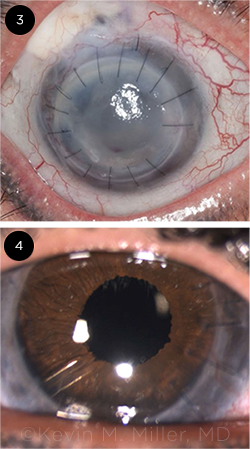 |
SIGNIFICANT DAMAGE. (3) At presentation, this patient had a blepharoptosis, a failed corneal graft, a failed trabeculectomy, and a failed tube shunt. The eye was completely aniridic. (4) After numerous surgeries—including a repeat PK, IOL exchange, implantation of the HumanOptics artificial iris, glaucoma tube shunt surgery, and blepharoptosis repair—the patient now has end-stage glaucoma, with a small island of remaining vision.
|
After explantation. The mean follow-up after explantation was 35.5 ± 38.1 months. Complications observed during this time included native iris defects (11 eyes, 45.8%), persistent glaucoma (seven eyes, 29.2%), cataract (five eyes, 20.8%), corneal edema (four eyes, 16.7%), and intraocular inflammation (two eyes, 8.3%).
Secondary surgeries included cataract extraction, IOL exchange, pupilloplasty, corneal transplantation, tube shunt implantation, endoscopic cyclophotocoagulation, and implantation of a medically indicated artificial iris (HumanOptics). A few of the patients had to undergo multiple surgeries, Dr. Miller said. “The problems go on for the rest of their lives—and many of these patients are in their 30s and 40s when we first see them.”
A clinician’s frustration. It is frustrating that these invasive ocular devices can be sold as an aesthetic accessory despite the documented risks, Dr. Miller said. “These have not been tested in vigorous clinical trials. Not in the United States, not in Europe, not anywhere.”
—Linda Roach
___________________________
1 Ghaffari R et al. Am J Ophthalmol. Published online Jan. 22, 2021.
___________________________
Relevant financial disclosures—Dr. Miller: None.
For full disclosures and the disclosure key, see below.
Full Financial Disclosures
Dr. Abràmoff Alimera: C; Digital Diagnostics: C,O,P; NovaGo: C.
Dr. Finger Liberty Vision: O.
Dr. Foster Aciont: S; Alcon: S,L; Aldeyra: C,S; Allakos: C; Allergan: L; Bausch + Lomb: C,S; Clearside: S; Dompé: S; Eyegate: C,S,O; Genentech: C; Novartis: C,S; pSivida: C,S; Mallinckrodt: S,L.
Dr. Miller Alcon: C; Johnson & Johnson: C,P; Lensar: C; Oculus: C.
Dr. Sun Adaptive Sensory Technology: S; Boston Micromachines: S; Genentech: C; Novartis: C; Novo Nordisk: C,S; Optovue: S; Roche: C,S.
Disclosure Category
|
Code
|
Description
|
| Consultant/Advisor |
C |
Consultant fee, paid advisory boards, or fees for attending a meeting. |
| Employee |
E |
Employed by a commercial company. |
| Speakers bureau |
L |
Lecture fees or honoraria, travel fees or reimbursements when speaking at the invitation of a commercial company. |
| Equity owner |
O |
Equity ownership/stock options in publicly or privately traded firms, excluding mutual funds. |
| Patents/Royalty |
P |
Patents and/or royalties for intellectual property. |
| Grant support |
S |
Grant support or other financial support to the investigator from all sources, including research support from government agencies (e.g., NIH), foundations, device manufacturers, and/or pharmaceutical companies. |
|
More from this month’s News in Review